17
____________________________________________________________
Approximately 13 million Americans suffer from arthritis, making it the nation's number-one crippler. Over 10 million have seen a doctor seeking relief and more than 3 million report limitation of their usual activity because of the disease. An estimated 1.3 billion dollars is the yearly toll on the economy (1).
Arthritis is not a killing disease, so the prevalence rises with age,the victims becoming disabled and wracked with pain -- but they continue to live and suffer. Arthritis gradually withdraws from productive activity large numbers of otherwise capable people.
Arthritis, rheumatism, and other related conditions are often referred to as the collagen diseases because of the definite involvement of this protein in their genesis and cause. Anyone having read the previous chapter on heart disease will recall the relation of ascorbic acid to collagen production and the absolute necessity for the presence of high levels of ascorbic acid in the body for the proper syntheses and maintenance of high-quality collagen protein. Briefly,collagen makes up about a third of our body's protein content. It is deprivation of ascorbic acid, with the consequent synthesis of poor quality collagen or no synthesis at all, which brings on the most distressing bone an joint effects of clinical scurvy. There can be no doubt about the intimate association of ascorbic acid and the collagen diseases.
Rivers (2), in 1965, in a review article on the tissue derangements caused by a lack of ascorbic acid states "Abnormalities" in this protein (collagen) are baic to the crippling deformities associated with rheumatic diseases and with a number of congenital connective tissue defects." Robertson (3), in studies on induced granuloma tissue of prescorbutic and normal guinea pigs, showed that guinea pigs deprived of ascorbic acid for only 14 days produced tissue containing only 2 to 3 percent collagen, while the tissues in normal guinea pigs contain 14 to 16 percent. Udenriend (4) Stone and Meister (5), and many others have shown that the dependence of high-quality collagen protein on ascorbic acid is due to its chemical action on one or two of the amino acid building blocks used in the manufacture of collagen.
As in many other diseases, the discovery of ascorbic acid inspired much research on the collagen diseases in the 1930s. A classic series of papers by Reinhart and coworkers (6) appeared in the period from 1933 to 1938 relating deficiencies of ascorbic acid and infection to the development of the rheumatoid process. They developed a theory intimately linking ascorbic acid with the genesis of rheumatic fever from the evidence of its social,urban, and familial incidence,the role of malnutrition,the age of incidence, seasonal incidence, geographic distribution, the symptomatic similarities of latent scurvy with the early rheumatic state,the role of infection, the problems of hemorrhage, and the existence of latent scurvy in rheumatics. Their logic was impeccable and everything fitted together like a jigsaw puzzle. They then confirmed these postulates by experimentally producing rheumatoid lesions in the guinea pig by combining ascorbic acid deprivation and infection. Infection alone did not produce these effects. It seemed that here, at last, was the answer to the age-old problem of the rheumatic diseases.
As might be expected, the publication of Rinehart's series of papers evoked much discussion and further tests. The papers resulting from this additional work may be divided into those that agreed with and more or less checked Rhinehart's work (7) and those that disagreed (8). Reviewing these discussions in detail now would serve no useful purpose and would occupy too much space. If anyone is interested, they can refer to the original papers. Of vital importance is the clinical work conducted, in these early days to test Rinehart's hypotheses; and we shall see with the advantage of hindsight how this clinical work was inadequate. We will first review the clinical work on dosages at the "vitamin" levels and observe their general ineffectiveness. After this we will take up the scant clinical data where tests were conducted using ascorbic acid at the lower fringes of megascorbic therapy with good clinical results.
M.P. Schultz (9) in 1936,reported on tests conducted at the hospital for the Rockefeller Institute in which ambulatory patients received from 100 to 250 milligrams of ascorbic acid daily either orally or intravenously for periods of months (the average was 2-1/2 months). The conclusion was that the incidence of rheumatic fever or the clinical manifestations of the disease were not favorably or demonstrably affected by this medication. F.H. Mosse (10),in 1938, described a single case, the dramatic improvement of a farmer with acute multiple arthritis, in the midst of a scurvy epidemic in China, by the ingestion of 800 to 1200 cubic centimeters of "fresh red fruit juice." He also discussed the etiology of rheumatic fever in northern China in those days. M.G. Hall and coworkers (11) a the P.B. Brigham Hospital in Boston reported, in 1939, that all of the patients with rheumatoid arthritis were placed on an intake of 200 milligrams of ascorbic acid per day for eight months with no improvement that could be attributed to this treatment.
In 1940, R.H. Jacques (12) reported that in a series of forty-eight arthritic cases, forty-seven had low levels of ascorbic acid in their blood plasma. Treatment with 100 milligrams a day of injectable ascorbic acid for one week and 300 milligrams a day of injectable ascorbic acid for one week and 300 milligrams a day of ascorbic acid orally for another few weeks brought up the blood plasma levels. The patients were followed for a period of three weeks to six months thereafter on a regime of 100 milligrams of ascorbic acid a day orally. His conclusion -- there was no marked clinical response even though the plasma levels had returned to normal. Twenty percent were moderately improved, 33 percent were slightly improved, and 47 percent showed no change or were worse. In a short report in a Russian journal, Vilyansky (13) treated thirty-nine patients with 200 to 300 milligrams of ascorbic acid intravenously per day. He reported that his tests showed his patients to be quite deficient in ascorbic acid and they responded well to the treatment. There was less pain, better mood, less swelling, and increased mobility in twenty-six of his patients. Eleven took longer to respond and two showed no effect. These two had been treated previously with salicylates. He states that in most cases three to five injections of ascorbic acid were sufficient to "liquidate" the attack of rheumatism.
Freyberg (14), in 1942, using fruit juices or ascorbic acid in amounts to maintain the blood plasma levels at "normal" levels in thirty-seven patients, found that "there was no evidence that the arthritis was better or that the course of the disease was different in any way whether or not the vitamin C deficiency was corrected." Trant and Matousek 15), in 1949, reported their experiences treating a series of eighteen arthritic patients at Chicago Presbyterian Hospital with 100 Milligrams of ascorbic acid daily. They concluded, "On the principle of good hygiene it is well to restore low levels of serum ascorbic acid to normal, but not with the anticipation that any improvement in the arthritis will result."
Rinehart (16), in 1943, in a paper entitled "Rheumatic Fever and Nutrition," reviewed the work of the previous decade and admitted:
While it has been shown that vitamin C does not exert a specific curative effect upon rheumatic fever it is likely that the frequency and severity of the hemorrhagic manifestations have been reduced. It is not known to what extent vitamin C or related factors might further protect the patient. Maintenance of rheumatic patients on adequate amounts of ascorbic acid will evidently not prevent recurrence of the disease.
The conclusions to be drawn from these early tests are that the measurement of ascorbic acid blood levels is not a good criterion for therapeutic effects and that the approach used by all these investigators was wrong. They were trying to correct a nutritional deficiency instead of treating a serious disease. The daily dosages required to raise the blood levels of ascorbic acid to what they considered normal were greatly below the megascorbic levels actually required to obtain a definite therapeutic effect in the collagen diseases. These early clinical tests were experiments in home economics rather than the thorough pharmacological testing of a new medicament.
Massell (17), in 1950, in a preliminary report on the use of 4 grams of ascorbic acid (1 gram four times per day) in seven young patients (five to eighteen years) for only eight to twenty-six days obtained rapid cessation of symptoms and stated, "Our observations suggest that ascorbic acid when administered in sufficient amounts possesses antirheumatic activity." He also mentions:
Previous therapeutic failure may perhaps be attributed to the fact that practically all investigators were thinking in terms of vitamin C deficiency and, hence,used doses of ascorbic acid considerably smaller than those used by us. ... It is possible that individual doses of more than 1 gram or total daily doses of more than 4 grams, if found harmless, may prove to be therapeutically even more effective.
The purpose of the publication of this preliminary report was to "stimulate further investigations of the therapeutic potentialities of ascorbic acid." Large-scale tests were never made to check these exciting results. The only further testings which were made are the following highly successful clinical tests reported in the 1950s by private investigators -- then we have silence.
Baufeld (18), in 1952, using individual intravenous dosages of 6 grams of ascorbic acid for acute and chronic rheumatism, observed "astonishing" results in some cases. He also noticed good response in lumbago, sciatica, and bronchial asthma. He stated that he believed he had found something which called for further testing. In 1953,Greer (19) found 8 to 12 grams of ascorbic acid, in combination with antibiotics, to be an effective antirheumatic fever measure in several serious cases. McCormick (20), in 1955, after offering a scholarly review of the literature dating back to the seventeenth century, showed the relationship of scurvy to the rheumatic diseases and stated that a number of his acute rheumatic fever cases were treated with 1 to 10 grams of ascorbic acid daily with a rapid and complete recovery in three to four weeks without cardiac complications. Similar results were obtained in incipient arthritis. Afanasieva (21), in a 1959 Russian paper, noted gains in 48 rheumatic fever women patients using 1.25 grams of ascorbic acid daily for twenty to twenty-five days in combination with other therapy.
If the government agencies and the publicly supported foundations interested in the arthritic diseases, had pursued these scant but provocative leads supplied by Massell and others in the 1950s, the past two decades may have seen the elimination of these collagen diseases as a major crippler of the population.
18
____________________________________________________________
If the aging process is looked upon as a chronic, 100 percent fatal disease from which everyone suffers and which is present at birth and continues with increasing ferocity throughout life, we have a logical viewpoint to start our discussion. The first conclusion we can draw is that treatment of this chronic disease should not be directed against the acute symptoms developing in the later years but should be in prophylactic, preventative measures starting at birth and continuing throughout life.
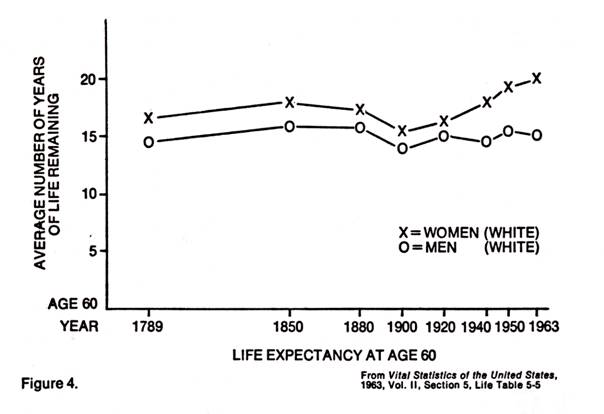
Further, if we look at some statistics on the human life span, we find some startling facts. Modern medicine can take credit for the rise in life expectancy at birth of nearly twenty years from the 49.2 years in 1900 and much more from earlier days (it was 38.7 in 1840). This stems from the drop in infant mortality and reduction of morbidity of childhood diseases. But, as pointed out by Bjorksten (1), in 1965, the life expectancy for those at age sixty has been practically the same since 1789 (Figure 4.). Medicine has not done much to prolong the life span for those who survive the early hazardous years.
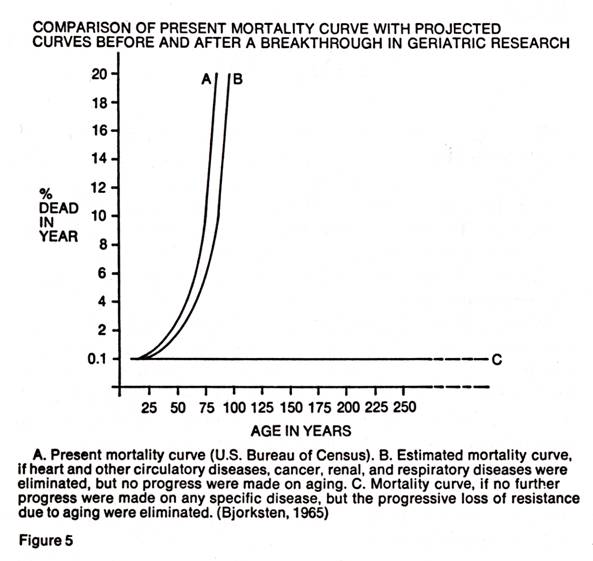
Bjorksten (1), in 1963, also compared the present mortality curves with a projected line which would be obtained if medical research were able to eliminate the progressive loss of resistance due to aging. The line was stopped at age 300 because there was no more room on the graph.
It was also recently noted by this author (2) that the current statistics on the human life span do not give a true picture of potential longevity because the "normal" population (which would be represented by lines A and B in Figure 5) used in the calculations of these statistics was suffering from uncorrected hypoascorbemia. Statistics based on a population of fully corrected individuals could be entirely different. The importance of the proper synthesis and maintenance of the vital protein, collagen, as a prime factor in inhibiting aging was also indicated. This synthesis and maintenance is wholly dependent on ascorbic acid.
The author believes that it is now practical to travel along line C of Figure 5 by the full "correction" of the genetic disease, hypoascorbemia, throughout life. The only tests needed are to see how far along line C we can travel. It is also his opinion that the proper use of ascorbic acid throughout life may provide the long-awaited breakthrough in geriatrics. Perhaps most importantly, ascorbic acid should also prolong the period of vigorous and healthy maturity, not merely prolong the life span.
The current theories relating to aging are backed by a substantial volume of published papers of which we can cit only few. The work of F. Verzar, W. Reichel, F.M. Sinex, D. Harman, I.G. Fels, and J. Bjorksten (1,3) indicates that senescence is due to profound changes in the elastic and other properties of the various environmental factors such as oxidation, free radicals, radiation, cross-linking, stress, and others,combined with time. Their research indicates that collagen is a very important factor in the aging process.
Here we are back again on the collagen-track with all the implications of the basic involvement of ascorbic acid in maintaining the collagen molecules in good repair and "young."
Many other reports of tests on the aging of the collagen macromolecule have confirmed the suspicion of its direct involvement in aging (4) (F.M. Sinex, 1957, A. Aslan and A. Vrabiesco, 1965; F. Verzar and H. Spichtin, 1966; C.D. Nordschow, 1966; R. Goodman, 1970, and many others). The extensive bibliographies given in these published reports indicate the vast amount of research expended in this field.
The use of antioxidants has been suggested many times to counteract the cross-linking and aggregative effects of oxidation and free radicals on the collagenmoleculrs. The 1968 paper by Tappel (5) reviews this subject and points out that the animal body, with its many oxygen-labile components, could not exist in this harsh oxidative environment without the presence of biological antioxidants that also serve as free-radical scavengers. Ascorbic acid is intimately involved in this biochemical scheme of natural fat-soluble and water-soluble antioxidants and he states, "Optimum amounts of vitamin C would be important in any attempts to slow the aging process."
Dr. Alex Comfort, speaking at the Eighth International Conference of Gerontology, also voiced the opinion that antioxidants may retard the aging process(6).
The comprehensive paper by Sokoloff and coworkers (7) at the Southern Bio-Research Institute showed, among other things, that blood-lipid abnormalities increased with advancing age and that ascorbic acid at 2 to 3 grams per day for twelve to thirty months improved this condition in 83 percent of their group of sixty cardiac patients. The 17 percent that showed no effect may have been helped had their hypoascorbemia been fully corrected by the use of more ascorbic acid daily. They also note the need for maintaining the ascorbic acid in the blood and tissues in the antioxidant form by the use of high daily intakes because the oxidized form, dehydroascorbic acid, has undesirable reactions.
There are so many references in the medical literature showing that ascorbic acid requirements are increased in old age and that the elderly suffer from serious depletion, that only a small sampling can be quoted here (8). Yavorsky, Almaden, and King, in 1934, showed that the ascorbic acid content of human tissues decreases with age. The ages varied from one day to seventy-seven years in five groups and the tissues examined included the adrenals, brain, pancreas, liver,spleen, kidney, lung, heart, and thymus. A substantial drop was shown in all cases. Rafsky and Newman, in 1941, examining twenty-five so-called normal individuals, aged sixty to eighty-three, found only two whose ascorbic acid retention behaved normally. Thewlis and Gale concluded, in 1947, that ascorbic acid deficiencies were common in older patients and that:
If there is any chance that a patient may have a cerebral hemorrhage or coronary occlusion, as indicated by high or fluctuating blood pressure, 500 to 1,000 milligrams of ascorbic acid should be given daily, parenterally, for several days.
In a follow-up study published in 1954 of 588 San Mateo County residents over fifty, one of the conclusions of Chope was that low ascorbic acid intake appeared to predispose the sample group to a high mortality. This was confirmed in the 1956 paper by Chope and Breslow. In a comprehensive study on the Nutritional Status of the Aging in California which correlated serum ascorbic acid and intake as reported in 1955 by Morgan and coworkers, good correlation was obtained, but the reported blood serum ascorbic acid values seem to high, indicating that some constant experimental shift to higher values operated during the test. This may be the result of the method they used for the determination of ascorbic acid in the blood serum which includes, besides ascorbic acid,the oxidation products of ascorbic acid itself. This well-planned study should be repeated using an analytical technique which would be utilized to differentiate the presence of reduced ascorbic acid from that of the oxidized form, dehydroascorbic acid, and other decomposition products. This same criticism on the choice of analytical methods applies to many other studies appearing after 1943, when these new analytical techniques were introduced (8).
other references (9),indicating higher ascorbic acid requirements in the elderly and lower levels found in the body,are Dawson and Bowers in 1961; and Bowers an dKubik, in 1965; Smolianskki, in 1965; Andrews and coworkers, in 1966; O'Sullivan and coworkers, in 1968; Mitra, in 1970; and many more references contained in the bibliographies of these papers.
One paper in this series which should be given special attention is that of Slotkin and Fletcher (10). This paper discussed the stresses of urologic surgery, especially prostatic surgery in patients in their 70s and 80s. Slotkin and Fletcher note that atypical bronchopneumonia is a common and often fatal complication of these operations. These postoperative complications are not truly pneumonic in character, but are the so-called wet chest and foul expectorations due to capillary secretions. They obtained good results,some spectacular, in spite of the pitifully small doses of ascorbic acid employed and concluded: "irrespective of the blood levels or deficiency of vitamin C, ascorbic acid is a valuable adjunct in tiding these aged patients over their critical postoperative period."
Smolyanskii (11) studied the effect of ascorbic acid on the production of important hormones from the adrenal glands of a group of 144 persons aged 60 to 90 years. He found that both the ascorbic acid blood levels and steroid hormone production were low. A single injection of only 500 milligrams of ascorbic acid increased the urinary excretion of these hormones, indicating a rise in their production by the adrenal gland. Continuing these injections produced further rises in hormone production. It is likely that if these elderly persons had been receiving adequate ascorbic acid over the years,their hormone production would have been maintained at desired youthful levels. The work of Patnaik (11) also indicates a connection between ascorbic acid and aging.
We now have a background of years of highly suggestive research. Yet the crucial tests to determine the actual effects of optimal intakes of ascorbic acid on slowing the aging process have never been started. The genetic rationale for these optimal daily intakes of ascorbic acid for the full correction of the human inborn error of carbohydrate metabolism, hypoacorbemia, is now available (2). The tests would involve simply taking normally healthy age groups and maintaining them for the rest of their lives on ascorbic acid intakes sufficient to fully correct this genetic liver-enzyme disease under conditions of little stress (about 3 to 5 grams of ascorbic acid per day). The health, well-being and mortality of this ascorbic acid group would then be compared with similar groups which are solely dependent upon their foodstuffs as their exogenous source of ascorbic acid. The results in a few years may be startling. Time is of the essence in having these tests started. This is the first time that we are in a position to correct this ancient human genetic disease. Let us make the most of it.
19
____________________________________________________________
An allergy is an abnormal, excessive biochemical response by the body to the introduction of a foreign substance (allergen). It is a bodily defense mechanism which has gotten out of hand. This uncontrolled response manifests itself in many different ways, but they are all basically the same. If the allergen enters through the skin, it may cause rashes or other skin disorders,the so-called contact allergies. If the allergin is a drug or a certain food it amy cause digestive upsets and other systemic symptoms of drug or good allergies. Bronchial allergies are termed "asthma." the allergens can also be physical agents,such as heat,cold, or sunlight, which cause unusual, strong reactions in hypersensitive individuals. When the allergen is a skin graft or a transplanted organ, the phenomenon is known as "rejection."
Anaphylactic Shock in Animals
Much work was started in the early 1930s on ascorbic acid's use in anaphylaxis and allergies. Many research reports were published, starting around 1935, and the following is only an incomplete summary of some of the papers. Here, again, a great deal of confusion resulted because many workers reported complete inhibition of the anaphylactic syndrome in experimental animals by ascorbic acid while many others reported no effect. Anaphylaxis or anaphylactic shock is an experimental technique used with test animals because it duplicates the human response in allergies. An animal is given an injection of a foreign substance and, after a suitable incubation period to allow the body's defense mechanism to respond, it is given a second injection of a minute amount of the same foreign substance. The biochemical response of the animal may be so violent that is may quickly die in anaphylactic shock. A review of this early work is found in the 1938 paper by Raffel and Madison, and by Walzer (with ninety references), citing the confusing results of the various workers up to that time (1). Because of the many experimental variables, both controlled and uncontrollable in these tests, many further papers tried to bring some order out of the chaos by involved techniques. Pacheco and coworkers,in 1938, concluded that ascorbic acid has a certain protective action against anaphylactic shock in the guinea pig. Yoshikawa, in 1938, came to a similar conclusion when large doses of ascorbic acid were continuously used, but thought that small doses seemed to increase allergic manifestations (2).
The 1940 paper by Yokoyama (3), of the Kitasato Institute, did much to clear up the importance of the ascorbic acid dosage in preventing death from anaphylactic shock. He took guinea pigs weighing between 200 and 300 grams an sensitized them to horse serum. Three weeks later, he injected them with a minute amount (the minimal lethal dose) of the same horse serum and within a few minutes all the guinea pigs were dead in anaphylactic shock. In other groups of sensitized guinea pigs, he injected ascorbic acid immediately before the same second shocking dose of horse serum. He are his results as quoted from his paper:
Thus when 5 to 10 milligrams of ascorbic acid was injected 2 to 3 minutes before the serum injection shock was not prevented; 20 milligrams delayed death from shock; 30 milligrams prevented shock symptoms and at times prevented shock death, whereas 50 milligrams prevented shock symptoms as well as death from shock in all cases.
If Yokoyama's figures are extrapolated for comparison to the 70-kilogram body weight of an adult human, his ascorbic acid dosages become the following: 2,800 milligrams are completely ineffective; 5,600 milligrams will delay death; 8,400 milligrams will prevent the shock symptoms and sometimes prevent death; 14,000 milligrams will prevent shock symptoms and death and will desensitize the case. This work was confirmed in part by Guirgis,in 1965, and by Dawson and West (3).
The Hungarian workers, Csaba and Toth (4), in 1966,were not able to confirm the is work in dogs, but this may have been because they used a shocking dose of horse serum, about 20 to 40 times more than the carefully worked out minimal lethal dose used by Yokoyama (3). Herxheimer (4), in 1965, was also not aware of Yokoyama's effective dosage levels when he reported no antianaphylactic effect for ascorbic acid at 10 and 20 milligrams per kilogram of body weight.
Hay Fever or Pollinosis
Now let us turn to allergic manifestations in man and look into hay fever, or pollinosis. In 1942 a paper by Holmes and alexander (5) appeared and gave the results of tests on twenty-five hay fever patients tested consecutively with 100 milligrams of ascorbic acid per day for the first week, 200 milligrams daily for the second week and finally, 500 milligrams daily for the third week. In most cases, little or no relief was afforded by the 100 milligrams per day level, but when the higher doses were used on the same subjects, they reported a high degree of success, only two of the subjects reporting "no relief." One of the subjects broke out in a rash and quit the test. Holmes extended this work to food allergies and, in 1943, published his results on 27 patients indicating 80 percent success with 500 milligrams of ascorbic acid a day. He notes that while ascorbic acid in nontoxic, he did observe several cases out of a large number where the patients suffered headaches or sore spots around the mouth and, in one instance, diarrhea.
Apparently there is a low percentage in the population of individuals hypersensitive to ascorbic acid who show these reactions to ascorbic acid even though Korbsch (5), in 1938, reported that ascorbic acid in oral doses up to 1 gram a day relieved serum rashes, erythema multiforme (a type of skin rash), and allergic coryza. A possible way of avoiding these reactions may be to build up gradually to the high dosage intakes rather than starting directly with the high levels.
Pelner (6), in 1944, showed that an extremely sensitive ragweed patient could be protected against adverse reactions to pollen-antigen injections by incorporating 100 milligrams of ascorbic acid with the injection. Pelner had also found previously, in 1943, that he could similarly prevent adverse reactions in a series of 51 patients to sulfonamide injections and, in 1942, he prevented the allergic reactions of a rheumatic fever patient to salicylates. Two other papers by Hebald and Englesher (7) appeared in 1944. Both claimed that ascorbic acid at 500 milligrams per day is not an effective treatment for hay fever. From these contradictory reports, it is evident that 500 milligrams a day is just marginal in hay fever treatment, giving the typical good results with some investigators and outright failures with others. From the lessons learned in Yokoyama's (3) anaphylactic tests on guinea pigs, it is likely that the higher levels of megascorbic therapy would produce more consistent and successful results.
Ruskin, in 1945, concluded as a result of his studies that ascorbic acid plays a valuable role in treating allergies at an optimum dosage of 750 milligrams daily either orally or by injection. In some cases the ascorbic acid therapy alone proved superior to the pollen desensitization used previously. A paper by Friedlander and Feinberg, appearing in 1945, concluded also indicated that 500 milligrams of ascorbic acid daily was insufficient to change the clinical course of hay fever and asthma (8).
Ruskin, 1947, published another paper reporting that sodium ascorbate was more effective than ascorbic acid in refractory cases of allergy and asthma at 1,200 to 1,500 milligrams per day. In 1948, Ruskin published another paper along similar lines and indicated additional successful results. In a study conducted in both Boston and New York on sixty hay fever patients given 1,000 to 2,250 milligrams of ascorbic acid daily along with a few milligrams of vitamin B1. as reported in 1949, Brown and Ruskin concluded that about 50 percent of their hay fever patients on the higher doses showed improvement. They stated, "The larger dose may have played a part in producing the apparently greater improvement in the larger percentage of patients." In this test series, one subject reported a laxative effect, two reported flushing and headache,and one reported a rash around the eyes and the scientists stated, "Approximately 5 percent of the patients may suffer mild, although easily controlled, side reactions" (9).
The reader now has a representative review of the clinical review of the clinical research on the use of ascorbic acid in the treatment of hay fever at levels from 100 milligrams to 2,250 milligrams a day. It shows the confusing results at the lower levels of treatment and the greater percentage of success as the dosages were increased. Yet in all these tests the dosages of ascorbic acid used were much below the levels of ascorbic acid indicated by current calculations to be synthesized in the liver of an equivalent-sized mammal under equivalent stress. No one in all these years has been inspired to test dosages of ascorbic acid more closely related to these mammalian levels in spite f the suggestive results of previous clinical tests that the degree of success was dose-related. The protocols of any future clinical tests on hay fever season (with and without other antihistamines). The seasonal dosage would be adjusted, depending on the results obtained. If hay fever sufferers were to organize and make enough noise, these tests would be conducted.
Asthma and Bronchospasm
The history of the use of ascorbic acid in the treatment of asthma also dates back to the mid-1930s and is also confusing. It was reviewed in the 1941 paper by Goldsmith (10), who noted the typical pattern of good results. Goldsmith measured the blood ascorbic acid levels of twenty-nine asthmatics and found twenty-two to be below 0.6 mg % (0.7 mg % is considered the minimal normal level) and in two of their patients with hay fever only, ascorbic acid was practically absent from their blood (0.07 and 0.08 mg %). On a regime of 300 milligrams of ascorbic acid daily for 1 week, 200 milligrams daily for the second week, and 50 milligrams daily thereafter, six of seven of their asthmatics were unable to maintain blood levels of l.0 mg %, which was easily achieved by a healthy control group. They interpreted this as a sign that asthmatics had a greater requirement for ascorbic acid. In some of their patients, they found a relationship between the low blood levels of ascorbic acid and the frequency and severity of asthmatic attacks.
Ten years later, in 1951, the literature was again reviewed by Silbert (10). Of the nineteen papers reviewed, thirteen reported benefit, some to complete remission of symptoms, while 6 reported little or no benefit. Silbert suggested that some of these failures may have been due to inadequate dosages of ascorbic acid.
A series of important papers reporting the work of W. Dawson and coworkers (3, 11) on the nature of the antagonism of ascorbate on bronchospasm and on the action of ascorbate on smooth muscle, appeared from 1965 to 1967. They showed that spasmogen-induced broncho-constriction in guinea pigs could be prevented by ascorbic acid. They believed this was due to a direct action of the ascorbate on the bronchial smooth muscle. They also showed that this action is dose-dependent; at low levels it may potentiate the effect of spasmogens, such as histamine, and at higher concentrations it inhibits their spastic effects. This dose-related smooth muscle phenomenon may explain some of the conflicting clinical results of the past four decades.
The protocols for future clinical research using ascorbic acid in asthma should include the megascorbic prophylaxis levels. The dosages would be increased to a point where a therapeutic effect would be obtained. In severe asthmatic attacks, large doses of sodium ascorbate administered intravenously should be tried to relieve the attack. For the safety of this procedure, check the references in Chapter 20 on eye conditions, where doses of 70 grams of sodium ascorbate have been used intravenously without undesirable side effects in the treatment of glaucoma.
Organ Transplants, Skin Grafts, and Rejection
When an organ is transplanted into a body, or even when a piece of skin is grafted onto a damaged surface, there is a very critical initial period of waiting to determine if the organ or the graft "takes." Frequently, there is the possibility that the body may consider the new organ or graft as a foreign substance and begin the allergic, immunological process that is known as rejection. The rejection phenomenon has serious consequences if a vital organ is involved and it may mean quick death for the individual or in a skin graft, death to the grafted tissue.
To inhibit the rejection phenomenon, medicine now uses large doses of radiation alone or in combination with the long-term use of various highly toxic, immunosuppressant drugs. Both radiation and these highly toxic drugs are additional biochemical strains on the patient who had undergone complicated surgery. The ascorbic acid levels in these patients, if they were ever measured,would probably be extremely low. These patients, in addition to their other problems, are likely to be suffering from a severe case of uncorrected hypoacorbemia resulting from the stresses of surgery, radiation, and toxic drug administration. Up to the time of this writing, I have been unable to find any reference to the use of large doses of ascorbic acid in the treatment of these patients, either a nontoxic immunosuppressant or merely to relieve their hypoascorbemia.
Here is a completely unexplored field in organ transplantation and skin grafting which might ensure the survival of these patients. In view of the known potential of ascorbic acid in wound healing and of its antiallergic effects when used in the proper large doses, there should be a high priority for tests of massive levels of ascorbic acid to prevent the rejection phenomenon. Animal tests should be started quickly and followed by tests on human transplants.
Protocol for clinical research in this area should include the long-term preoperative daily use of 5 to 10 grams of ascorbic acid, building up to the intravenous use of sodium ascorbate at doses up to possibly 100 or more grams per day intravenously during the postoperative immuno-suppresive phase. If the transplant or grafts "take" under this megascorbic therapy, it may be possible to reduce the ascorbate to a lower holding level.
If this works, it could lead to other valuable pathways, such as the use of high levels of ascorbate in the storage and preservation of organs and the possible use of nonhuman organs to relieve the shortage of human donors.
20
____________________________________________________________
Of all the disorders afflicting man, blindness causes the most widespread disability. Aside from the cost in terms of economic loss and the personal expenses of family care and dependency, the annual bill for aid to the blind approaches a billion dollars. It is estimated that a million people in the United States have visual impairment so severe that they cannot read a newspaper. Yet, in spite of significant advances in eye research, the incidence of blindness is increasing. Megascorbic therapy might one day help to reverse this trend.
Structurally, the eye is a spherical camera aimed by exterior muscles. It has a transparent window in the front (cornea) composed of a special protein and a large optic nerve exiting at the rear. The interior is divided into two chambers separated by a flexible lens which focuses the image on a thin, biochemically active membrane (retina) which transforms light energy into nerve impulses. These nerve impulses are gathered into the optic nerve and transmitted to the brain where the color pictures are "seen" and recorded. As would be expected of an organ of such biochemical activity, the eye was early found normally to contain high levels of ascorbic acid and seemed to have the ability to extract it from the blood and to concentrate it for its many vital functions.
The 1962 paper by Heath (1), with forty references to the literature, reviewed the work on ascorbic acid and the eye. He cited twelve separate biochemical processes in which ascorbic acid is involved and speculated on the functions of ascorbic acid in the eye and its possible involvement in diabetic retinopathy, detachment of the retina, and maintenance of the proper consistency of the internal fluids of the eye. It has been known since the early 1930s that ascorbic acid is normally found in the eye at much higher levels than in the blood and in many other tissues. Heath confirmed this by showing that the ascorbic acid levels in different bovine eye tissues were (in milligram percent) the cornea, 30; corneal epithelium, 47 to 94; lens, 34; retina, 22; and were higher than in the skeletal muscle, 2; heart, 4; kidney, 13; and brain 17' but were not as high as in the adrenal gland, 97-160; or the pituitary gland, 126. He states:
Animals which are capable of synthesizing their own ascorbic acid usually have tissue levels approaching saturation. It would, therefore, seem desirable to ensure that the intake of ascorbic acid by man is sufficiently high for tissue saturation. Lower intakes, although not leading to scurvy, may affect some metabolic processing in which ascorbic acid is involved.
Glaucoma
Glaucoma usually appears in middle life and is the second leading cause of blindness in the United States. High pressure within the afflicted eyeball eventually destroys the nerve cells within the retina and progressive loss of vision results. Glaucoma as present in about 2 percent of the population over forty,and 8 to 10 percent over sixty-five. It brings creeping blindness to 3,500 Americans a year.
The prevention of glaucoma is achieved by merely maintaining low intraocular pressure during the lifetime of the individual. The treatment of the disease, once it occurs, is to endeavor to reduce the intraocular pressure to normal levels to prevent further nerve damage. About a million Americans over forty years of age have glaucoma without knowing it. Many cases go undetected for years in spite of the availability of a simple, rapid, and painless tonometer test procedure. Control and prevention of the disease in its early stages is preferable to waiting for the agony of acute glaucoma to strike.
There was a period of intense research activity from 1964 to 1969 on the use of megascorbic levels of ascorbic acid or sodium ascorbate for reducing the intraocular eye pressure. Linner (2), in 1964 in Sweden, showed that 0.5 grams of ascorbic acid administered twice daily produced a significant drop in the intraocular pressure of normal eyes. He published another paper, in 1969, in which he showed that 2 grams of ascorbic acid a day, orally, produced the same significant decrease in glaucomatous eyes.
The year 1965 saw the beginning of a four-year period when numerous papers reported on the prompt reduction of the intraocular pressure, with no side effects, by the intravenous injection of 20 percent sodium ascorbate solution at doses of about 70 grams per treatment. Virno and coworkers (3) in Rome published five papers in this period, the group from the University of Rome's Ocular Clinic (4) presented seven papers, one came from Switzerland (5), and one from Finland (5). Even though two papers were published in American journals in 1966 and 1967 by the Italian workers (3), no papers coming from American authors could be found on this exciting line of research.
Such a research silence on the part of American scientists can only be interpreted as an indication that no work has been carried out in the United States in the past six years in this field. Yet, during this same time, numerous government bulletins have appeared describing the urgent need for solving the problem of glaucoma and the daily mail is filled with repeated requests for donations to eye research charities. Where is the money going? What is being done with the available funds?
Research should be started immediately on population groups near forty years of age and older to determine the long-term effect on the inhibition of glaucoma by means of the continued daily intake of about 3 to 5 grams of ascorbic acid. The use of higher dosages, both orally and intravenously, for the therapy of incipient and advanced glaucoma should be included in the research protocols. This will help to determine if a simple and harmless ascorbic acid regimen can be worked out which will prevent blindness in our senior citizens.
Cataracts
A Public Health Service government bulletin (6) starts the discussion of cataracts with:
Cataracts are the leading cause of blindness in this country. They occur when the chemical composition of the crystalline lens changes, making it opaque rather than transparent. When cataracts form, the only way to restore sight is to remove the afflicted lens. In the majority of cases, cataracts appear to be part of the aging process. Uveitis (inflammation of the eye) and physical and chemical injury are other causes.
Let us discuss these authoritative statements individually:
1. That cataract is now the leading cause of blindness there is no argument -- but need it be? The proper long-term use of ascorbic acid may have a profound effect in reducing the incidence of this condition and preventing blindness.
2. Changes in the chemical composition of the lens makes it opaque -- correct, no argument. But what is the chemical composition of the lens? It is made from a specially oriented helical protein (7). Dische and Zil (8), in 1951, start their paper, "The most striking chemical change in the lens during the cataractous process is the decrease in sulfhydryl groups." Sulfhydryl groups, like ascorbic acid, are strong, normally occurring reducing agents, and are destroyed by oxidative processes. Possibly, the high levels of ascorbic acid found in the normal eye are there to protect against the loss of these sulfhydryl groups by oxidation. Studies in India (9), from 1963 to 1969, where senile cataract is rampant, occurs at an early age, and matures more quickly, show that cataractous eyes have a much lower content of ascorbic acid than normal eyes. One of these papers (Nema and Srivastava) suggests that the chronically low ascorbic acid content may be responsible for the high incidence of senile cataract.
3. When cataracts form, the only way to restore sight is to remove the afflicted lens -- right and wrong. This is the opinion of many present-day ophthalmologist. While some research shows that it is possible to slow down the cataractous process, no work could be found which would indicate that the proper use of ascorbic acid has been tried to reverse the cataractous process.
4. In the majority of cases, cataracts appear to be part of the aging process -- right. But let us do something about this by inhibiting aging (see Chapter 18).
5. Uveitid and physical and chemical injury are other causes -- right. All these stresses reduce the ascorbic acid levels in the eye. The 1941 paper f Lyle and McLan of the Royal Air Force on corneal inflammations should not be ignored. They stated:
Treatment by means of ascorbic acid intravenously is of therapeutic value. The improvement in most cases is almost dramatic. In most cases there is no reason to believe that a general vitamin C deficiency exists. It appears, therefore, that the beneficial results are obtained by flooding the bloodstream with excess of ascorbic acid.
This work was confirmed by Summers in 1946. The profound effects of ascorbic acid on the healing of deep corneal ulcers caused Boyd and Campbell, in 1950, to state and recommend, "We therefore suggest that ascorbic acid, in such massive doses as 1.5 grams daily, has a value in therapy apart from its normal role as a vitamin at accepted levels of intake." The additional work of Campbell and coworkers, in 1950, and Boyd, in 1955, on experimental eye burns, supplies additional confirmation for the need for adequate levels of ascorbic acid in the eye for recovery from heat injury 10).
The answers to this discussion of cataracts seem to be supplied by ascorbic acid. Are they not sufficiently suggestive to warrant further research and investigation?
The literature cited in this discussion of cataracts is but a small fraction of the total which has been published on ascorbic acid and the eye since the early 1930s. To thoroughly review this voluminous work is beyond the scope of a short monograph. We have to omit the work done on experimental diabetic cataracts, naphthalene cataracts, and dinitrophenol cataracts. But before closing this chapter, let us consider only four of the papers on senile cataract.
As long ago as 1939, Muhlmann and corworkers (11), in the Argentine, obtained 90 percent good results in sixty patients with 113 incipient senile cataracts by 2 series of daily injections, for ten days each, of 50 to 100 milligrams of ascorbic acid. He concluded that the treatment had no contraindications, should be tried in all incipient cases, and is more effective the earlier it is used.
In another 1939 paper, "Vitamin C and the Aging Eye," Bouton (11) of Detroit found "ascorbic acid deficiency can be held partly responsible for impairment of vision associated with senescence of the human eye and that the administration of ascorbic acid by mouth can counteract this process." He gave 350 milligrams of ascorbic acid a day for four to eight weeks and obtained improvement in vision in 60 percent of the treated group; marked improvement usually set in within the first two weeks of treatment. He believed that cataracts already formed were not affected and the benefits obtained were due to clearing of the other optic media and to some degree to a beneficial effect on the retinal vessels and the head of the optic nerve. While 350 milligrams of ascorbic acid a day was considered a huge dose in 1939, the administration of multigram daily levels would have obtained even better results.
Atkinson, an ophthalmologist of more than thirty years' experience, published in 1952, a scholarly paper on the senile cataract (11). He stated,"...in a larger percentage of cases than most surgeons have realized , cataract is a preventable disease." In 1952 he had over 450 cases of incipient cataract under his treatment which included, among other dietary suggestions, the administration of about 1 gram of ascorbic acid a day. He noted that untreated incipient cataracts matured in four years or less, some taking only one year, Of his over 450 patients under prophylaxis, only a limited number matured and went to surgery, whereas formerly nearly all had to submit to surgery. He states that in a number of his patients the cataracts have remained incipient over a period of eleven years.
The promising leads relating to ascorbic acid cited above, have not been picked up or been the subject of intensive research in an effort to help prevent this annual plague of blindness. Why? A search of the government bulletin (6) entitled, "Research Profile -- Summary of Progress in Eye Disorders," discussed before, fails to reveal a single mention of ascorbic acid in its 16 pages. This indicates that no research on the use of ascorbic acid for the prevention of blindness is being conducted at the National Institutes of Health or the National Institute of Neurological Diseases and Blindness. The same situation probably exists in the research facilities of the many publicly supported charitable foundations for the blind.
Most of the investigators using ascorbic acid in the treatment of eye pathology employed it orally or by injection. It is also possible to use it as a solution of sodium ascorbate applied topically. This is especially effective when the topical application is done iontophoretically. This method uses a harmless mild electric current to force the ascorbate into the eye tissues. As pointed out by Erlanger (12), in 1954, after many years of research, iontophoresis is another neglected principle of therapy which should find much wider use in the treatment of eye diseases. Topical megascoric therapy and iontophoresis should be a most valuable combination.
Retinal Detachment
Another area for eye research is in retinal detachments. A 1964 paper by Weber and Wilson (12) showed that the ascorbic acid levels in the subretinal fluid decreased with the length of time of the retinal detachments. Possibly, individuals on high levels of ascorbic acid would have less chance of suffering retinal detachment. The research on this condition could be combined with the above suggested tests on glaucoma and cataracts to determine whether the prophylactic daily dosage of 3 to 5 grams of ascorbic acid would also reduce the incidence of retinal detachments.
21
____________________________________________________________
Peptic ulcers may be the butt of many jokes, but as any ulcer sufferer can testify, having one is not funny. Ulcers are a painful, chronic disease affecting about 14 million Americans during their productive years. Every day some 4,000 individuals develop an ulcer and each year about 10,000 people die of complications from peptic ulcer. The drain on the economy is estimated to be 500 million dollars in lost man-hours and cost of medical treatment. A simple, inexpensive, successful preventive and therapeutic regime is needed.
Our stomach is the second stop in the processing of food for digestion and absorption. It is a tough, strong, muscular, spheroidal-shaped bag with an opening at the top (inlet) and at the side (outlet). Each opening is surrounded by a circular muscle, the sphincter, controlled by nerve impulses for opening and closing. The top opening is connected to the esophagus, which carries the food from the mouth; the side opening is connected to the duodenum, the first section of the intestines. The lining of the stomach secrets strong hydrochloric acid and a powerful enzyme, pepsin, which dissolves and digests proteins.
The stomach contents are normally highly irritating, corrosive, and erosive. This can be seen when the stomach contents sometimes back up into the esophagus producing the distressing sensations of "heartburn" and in the sour, irritating taste of vomit. Since the stomach walls are themselves made of protein, they must in some way be protected against the corrosive action of their own secretions. Sometimes this equilibrium is disrupted and open sores and lesions in the lining result. If they are in the stomach, they are called gastric ulcers; if in the adjacent intestine, duodenal ulcers. In the United States, duodenal ulcers are about eight times more common than gastric ulcers.
The secretion of hydrochloric acid and the enzyme, pepsin, is also under nervous control. The stimulation of these nerves is caused by food entering the mouth or even by the thought of food, so that the stomach will be ready for processing the food when it reaches there. In nervous people, smokers, excessive drinkers, or individuals under stress, this nervous stimulation does not turn off at the proper sequence or turns on when there is no food in the stomach. When no food is present to take the full brunt of the corrosive chemical attack of the stomach juices, gastric distress is felt and, if long continued, actual attack of the lining may result.
Animal experiments have been conducted since the early 1930s on ascorbic acid and its relation to gastric and duodenal ulcers. In 1933, Smith and McConkey (1), working in a New York State tuberculosis hospital, performed autopsies on 1,000 guinea pigs that had been fed a normal diet and failed to find a single spontaneous stomach ulcer. Of seventy-five guinea pigs fed a diet deficient in ascorbic acid, twenty, or approximately twenty-six percent, developed ulcers. In eighty guinea pigs fed the same deficient diet but supplemented with added ascorbic acid, only one developed ulcers. In other experiments, they found that diets deficient in vitamins A, B, and D did not produce ulcers if the ascorbic acid supply was adequate. Mechanical injury to the duodenum lining of guinea pigs fed an adequate diet was followed by rapid and complete healing, while similar injury to guinea pigs on an ascorbic acid-deficient diet resulted in the formation of duodenal ulcers. They also gave a small group of their tuberculous patients with chronic duodenal ulcers tomato juice supplements (their only source of ascorbic acid in those early days) with favorable responses. They also advised adding tomato juice or orange juice to the scurvy-producing Sippy or Lenhartz diets used for ulcer treatment. Hanke (1), in Germany in 1937, confirmed this work.
There is an extensive medical literature on clinical tests going as far back as 1934 correlating deficiency of ascorbic acid with high incidence of gastric and duodenal ulcers, bleeding ulcers, and poor healing after surgery. Many of the papers point out that the ulcer patient should receive adequate amounts of ascorbic acid.
Over the years, the author has collected over fifty medical research papers on this subject with no claim that this represents all the papers which have been published. Complete reference to all these papers is obviously beyond the scope and space of this book. Instead, a limited illustrative selection of twelve papers is included in the bibliography covering the period from 1936 to 1968 (2). The earliest papers show that ulcer patients have higher requirements for ascorbic acid than normal subjects. The patients have low, inadequate intakes of ascorbic acid and are in a state of subclinical scurvy and there is poor healing of the ulcers and the wounds after surgery. The papers recommend that ulcer patients receive plenty of ascorbic acid. The following are quotes from a few of these early papers:
It is important for the clinician to make sure that patients with peptic ulcers are receiving an adequate amount of vitamin C ... The severest degrees of vitamin C deficiency were found in the patients with haematemesis (vomiting of blood). It is suggested that large doses of vitamin C should be given to all subjects of peptic ulcerations and haematemesis in order to saturate them as rapidly as possible (2).
The results and suggestions contained in these early references have been repeated in the later papers and have continued up to the present time.
In 1968, Russell and coworkers (2) compared a series of sixty hospitalized patients with gastrointestinal hemorrhage -- 2 with peptic ulcer -- with a group having uncomplicated peptic ulcer and with healthy controls. They found significantly lower ascorbic acid levels in the bleeding group than in the uncomplicated peptic ulcer group, which was much lower than the healthy controls. The differences were more striking with advancing age over forty-five. They stated That only six of the bleeders had any clinical evidence of scurvy but that the ret suffered from a subclinical form of the disease. They believed this subclinical scorbutic state prevented healing of the bleeding ulcers and maintained hemorrhage in the gastric erosions precipitated by other factors such as aspirin or alcohol.
Certain drugs, such as aspirin, cortisone, and other anti-inflammatory agents, and cinchophen, are known to provoke ulcers and gastric hemorrhage. This is especially the case when a deficiency of ascorbic acid is present. In animal experiments, the administration of ascorbic acid along with the toxic drug reduced the incidence of peptic ulcer and gastric hemorrhage to such an extent that it prompted one author (Aron) to suggest, "Therefore it would seem judicious in human therapeutics to include ascorbic acid in every prescription for an anti-inflammatory drug" (3).
In any surgery, the importance of ascorbic acid has long been known (see Chapter 27). Patients undergoing surgery for ulcers are no exception. In a 1947 paper, Zerbini (4) discusses two surgical cases of patients with ascorbic acid deficiency. One patient exhibited severe surgical shock during the operation and the other patient showed no evidence of healing of the surgical wounds when the stitches were removed on the seventh postoperative day. This latter patient had been given a daily injection of 200 milligrams of ascorbic acid, but obviously this low, vitaminlike dosage was insufficient to supply the patient's high demands and as a result the wounds did not heal.
Williamson, in 1967, again confirmed the low ascorbic acid levels in patients subjected to gastric surgery and said that in these patients, "the administration of ascorbic acid would seem obligatory." Cohen, in the same year, stated that all patients with gastrointestinal disorders should be suspected of having subclinical scurvy. He also pointed out that this concept was proposed by Lazarus in 1937 but was "not yet generally acknowledged." This was over three decades ago -- the medical mills certainly grind slowly. Three other papers and a review added further confirmation to the pathogenetic role played by low levels of ascorbic acid in gastrointestinal disorders. In the paper by Cohen and Duncan they state:
[Patients should] be given routine ascorbic acid supplements before surgery and during the phase of early wound healing ... There are no known hazards of ascorbic acid therapy, and overdosage is therefore of no practical importance (4).
In a thirteen-page government bulletin (5) entitled "Peptic ulcer," prepared by The National Institute of Arthritis and Metabolic Diseases of the National Institutes of Health, there is not a single mention of ascorbic acid in the entire booklet. Nothing is said of its possible role in ulcer formation or in the ulcer treatment, in spite of the worldwide background of nearly four decades of research, some of which has been cited above. This is a bulletin sold to the general public for its information on the causes and treatments of this disease. "The Medical Letter," which is a semimonthly publication for doctors and is designed to convey authoritative recommendations for current medical treatments, devoted a large part of its December 26, 1969 issue to a discussion of the "Medical Treatment of Peptic Ulcer" (5). Here again no mention is made of ascorbic acid in the two and a half pages of discussion. It seems rather fantastic that, in both these publications, all of the suggestive work reported in the medical literature on ascorbic acid in ulcer therapy, can be so blatantly ignored. It also indicates that the use of ascorbic acid in ulcer therapy is not widely practiced and that ulcer patients are generally denied the possible benefits indicated in the above review of medical literature.
The following clinical research proposal is made in the hope that it will be picked up and tested by the government health agencies, by the publicly endowed health foundations with clinical testing facilities, or by doctors in the gastrointestinal field. The proposed rationale for the use of sodium ascorbate instead of ascorbic acid in ulcer prevention and therapy at megascorbic levels combines the antacid and buffering capacity of sodium ascorbate with its wound healing and antihemorrhagic effects. The research protocol would include the use of 0.5 to 1 teaspoonful (1.5 to 4 grams) of sodium ascorbate, dissolved in a glass of milk, taken before meals and at bedtime. For gastric distress at other times, 0.5 teaspoon of sodium ascorbate, dissolved in about 2 ounces of water, will usually provide immediate relief. This simple regime was very successful in several ulcer volunteers who were thus able to avoid surgery. The use of sodium ascorbate should be subjected to large-scale clinical testing to determine its value as a new approach to peptic ulcer prevention and therapy.
22
____________________________________________________________
It has been estimated that about 100,000 deaths occur each year in this country as a result of various diseases of the kidneys or their complications. Kidney and related diseases are the main cause of work loss among American women. About 3.5 million Americans have unrecognized and undiagnosed infections of the kidneys and urinary tract. Painful symptoms will prompt some to seek medical advice but others, a significant number, will have no warning until the damage has reached an advanced stage. An effective prophylactic regime is, therefore, urgently needed.
The kidneys are complex biochemical organs serving mainly to regulate and maintain our internal environment. They do this by varying the volume of the urine and the secretion into it of various bodily waste products which would otherwise pollute our system. The two purplish-brown, bean-shaped, depolluting kidneys are located in the small of the back and are equipped with a large blood supply. The renal artery brings the polluted blood to the kidney and the renal vein carries away the purified blood. The urine, produced by a complete filtration system in the kidney, is collected into a duct called the ureter, which carries the fluid to the bladder for storage. Another tube, called the urethra, carries the urine to the outside for disposal. Because of an evolutionary anatomical compromise of Nature, the final urinary structures are tied in with the reproductive organs, which complicates an already complex system. The entire structure from the kidneys to the exit tubes is called the genito-urinary tract.
The magascorbic treatment rationale for many urological, renal, or genito-urinary diseases would comprise the following steps and the body's resulting physiological responses. Large doses of ascorbic acid are administered, preferably orally an in solution, at frequent intervals. The doses will be of the order of 2 grams about every two hours. It is conveniently given by dissolving about a 0.5 teaspoonful of ascorbic acid in about four ounces of fruit or tomato juice or in about two ounces of water sweetened to taste with sugar or artificial sweetener. The ascorbic acid could also be given parenterally if the physician desires.
The ascorbic acid will be rapidly absorbed and will enter the bloodstream causing a rise in the ascorbic acid blood levels to above the kidney threshold. The kidneys start pulling it out of the blood and excreting it into the urine. This removal by the kidneys continues and before the blood is exhausted another large does of administered ascorbic acid maintains the excretory function at a high rate. The ascorbic acid level in the urine builds up to a concentration where it can exert bacteriostatic, bactericidal, and virucidal effects. Phagocytes in adjacent tissues would be stimulated to efficiently digest any bacteria.
Here, then, we have a situation where the complete genito-urinary tract from the renal tubules to the urethra would be continuously bathed in a fluid that is bactericidal and virucidal, and has detoxicating and wound-healing properties. Infections of the urinary tract should be more easily controlled through the use of this regimen. If antibiotics or other medicaments are employed,the ascorbic acid should aid and potentiate their effects. There do not seem to be any predictable contraindications to its use as an adjuvant treatment. The continued presence of high levels of ascorbic acid in the urine in contact with all these tissues should prevent any incipient infections from developing.
For urological surgery a suggested program could include maintaining the patient on a preoperative schedule of about half the suggested dosage level for a few days to a week before surgery. During and after surgery the dosage schedule should be maintained and continued until healing is complete. The patient may then put on a maintenance dose of about 5 grams per day. All these doses are only suggested starting points and may be varied as experience dictates.
A simple prophylactic regime to prevent the incidence and recurrence of kidney diseases could be based on the maintenance of high levels of ascorbic acid in the urine. This could be accomplished by the long-term use of ascorbic acid, about 3 to 5 grams a day in three to five spaced doses. Large-scale clinical testing of this simple regimen would provide the statistics to determine its usefulness.
BLADDER TUMORS
In 1969, Schlegel, Pipkin, and coworkers (1), at Tulane University School of Medicine, summarized the results of their research on bladder tumor formation. They demonstrated that the oral administration of large quantities of ascorbic acid, sufficient to produce a significant rise in the ascorbic acid content of the urine, will prevent the development of bladder cancer. They suggest the daily intake of 1.5 grams of ascorbic acid in three spaced doses for "individuals who due to age, cigarette smoking, or other factors, may be prone to bladder tumor formation."
RENAL FAILURE
Another area in which ascorbic acid may be useful and has been practically unexplored is in renal failure. When renal failure occurs,the kidneys malfunction and various chemical imbalances are produced in the blood which can be fatal. Heroic measures may be necessary to save the patient. One of the measures involves connecting the patient's blood system to an artificial kidney machine, if one is available, to take over the function of the damaged kidney. This is called "hemodialysis." The machine purifies the polluted blood and returns the purified blood to the patient. Until the kidney becomes normal again, the patient must make repeated trips to the machine in order to remain alive, which is a rather expensive and tedious procedure.
In 1968 and 1970, it was shown that, when patients are connected to the kidney machines, the hemodialysis not only removes the undesirable products from the blood but also removes a substantial proportion of the patient's already low stores of ascorbic acid (2). Replacement of his ascorbic acid is needed and page 1345 of the 1970 paper states:
Since ascorbic acid lost from the plasma during dialysis is not adequately replaced by dietary consumption of vitamin C, patients undergoing maintenance hemodialysis should receive ascorbic acid supplementation as an important part of their therapeutic regimen.
Further clinical studies should be conducted on this renal failure to determine the effect of continued daily megascorbic dosages, both orally and intravenously, on the detoxifying effects of ascorbic acid in relieving the build-up of toxic materials in the blood. If ascorbic acid can control this toxic burden, it might mean fewer trips to the scarce kidney machines with consequently less stress on the patient and his pocketbook.
Neglected but highly significant observations on rabbits with both kidneys removed, published n 1950 by Mason, Casten, and Lindsay (2), supply additional incentive for more research on the possible usefulness of ascorbic acid in kidney failure. Rabbits with both kidneys surgically removed uniformly die in three to four days. If, however, these animals without kidneys are injected with a mixture of ascorbic acid and p-aminobenzoic acid, the scientists state;
The duration of survival was strikingly increased, ranging from five and a half to eight and a half days. Even more striking was the improved condition of the animals during most of the period of survival. They were alert, active, and in most respects behaved like normal rabbits until a few hours prior to death.
In kidney transplantation the possibility of using megascorbic levels of ascorbic acid has never been adequately explored. The maintenance of these high levels may reduce the occurrence of rejection reactions and certainly would help ensure survival of the patient by counteracting the effects of surgical shock, promoting kidney function during recovery, and aiding in wound healing. The kidney research on ascorbic acid should be oriented toward the prevention of kidney damage by continued use of ascorbic acid, and if kidney damage is already present, to determine if biochemical repair can be effected. In this way, the expensive trauma of hemodialysis and transplantation could possibly be avoided. There are many publicly supported foundations and government agencies which could undertake this work.
Stone Formation
A criticism that has been leveled against supplying to humans the daily amounts of ascorbic acid which are normally produced in other mammals is that its acidifying effect on the urine might increase the incidence of stone formation. The formation of stones in the urinary tract is a very complex subject and intensive and large-scale research should be conducted to resolve this important question.
Abundant archaeological evidence indicates that the stone formation is among the oldest afflictions of man. It is common today in all parts of the world and in some areas of the world the incidence is so high that they are known as "stone belts." In the 1964 pater, containing a bibliography of 104 references, Gershoff (3) states, "Urinary calculi vary so much in form, occurrence, and composition that it is unlikely that a single mechanism is responsible for their production." The composition depends on whether it is a kidney stone or a bladder stone and in what part of the world the individual lives. About 1 percent of the 280,000 surgical cases admitted to the London Hospital from 1960 to 1935 were for stones of the urinary tract. In 25,000 autopsies at the University of Minnesota hospitals, the incidence of kidney stones was 1.12 percent. The incidence varies considerably in different parts of the world with a high percentage of bladder stones in children one to ten years old in Thailand, India, Syria, China, and Turkey. In an analysis of 1,000 urinary stones from the United States, 52 percent contained phosphates, 33 percent were calcium oxalate, 6 percent were urate stones, and 3 percent cystine. Because of the wide variation in composition, the acidifying effect of ascorbic acid in the urine may inhibit stone formation of certain types, especially those containing phosphates, which comprise a large fraction of those found in American urinary stones. McCormick (4), in 1947, surveyed the worldwide incidence of stone formation and his own clinical experience and experimentation, and he concluded that stone formation (urinary, salivary, biliary, and so on) was due to a deficiency of ascorbic acid. He pointed out that administration of ascorbic acid had profound effects on urinary sedimentation and crystallization. He states: "As soon as corrective administration of the vitamin effects a normal ascorbic acid level, the crystalline and organic sediment disappears like magic from the urine. I have found that this change can usually be brought about in a matter of hours by large doses of the vitamin -- 500 to 2,000 milligrams -- oral or parenteral. Subsequent maintenance doses of 100 to 300 milligrams, daily, are usually sufficient to keep the urine free from these deposits. It would thus appear that deficiency of vitamin C, which is the predominating dietary defect in the various 'stone area,' may provide the predominating factor in urinary lithogenesis [stone formation]."
Most people do not drink enough water and this is a neglected and little explored factor in stone formation. It is common knowledge that the tendency for salts to crystallize our of solution is greater the more concentrated the solution is. In people whose water intake is low, the urine is much more concentrated. In the "stone belts" of the world, water is scarce and of poor quality, and the climate is hot, so most of the population is in a chronic state of water deprivation. In Israel, where there is a high incidence of urinary stone formalin, Frank and coworkers (5) were able to reduce the incidence of stone formation by merely educating the settlers to drink more water. They stated, "Preliminary results, summarizing a 3-year period of study, suggest that education is capable of raising urine output and preventing urolithiasis (urinary stone formation) in a hot, dry climate." It is likely that copious daily intakes of good soft drinking water along with the high ascorbic acid intakes would tend to correct any individual tendency to stone formation. The author, who has been ingesting high levels of ascorbic acid for over three decades and has not been troubled with stone formation, also tries to drink at least a quart of water a day in addition to other fluids. Large-scale clinical studies should be started to obtain further data because there are probably millions of Americans taking high levels of ascorbic acid on their own volition.
Several minor studies on urinary oxalate excretion have already been made because oxalate formation is a possible result of ascorbic acid breakdown in the body and calcium oxalate is a constituent of many human stones. In a test of 51 male subjects reported in 1954 by Lamden and coworkers (6), results showed that the daily ingestion of up to 4 grams of ascorbic acid a day produced no significant increase in oxalate excretion. Eight grams a day produced an average increase of 45 milligrams a day and 9 grams increased average oxalate excretion by 68 milligrams a day. The range of urinary oxalate excretion of the subjects before taking the ascorbic acid was 10 to 64 milligrams per day. The normal individual variations among their subjects, of 54 milligrams a day, was thus greater than the average increase in excretion found for the 8-gram ascorbic acid test intake. Two papers from Japan in 1966 and one from Egypt (6), in 1970, reported similar additional results. Takenouchi and coworkers reported that 3 grams of ascorbic acid produced no marked increases in oxalate excretion in three subjects, while 9 grams a day increased it to 20 to 30 milligrams a day. The variation in oxalate excretion of their three subjects before taking the ascorbic acid (11 to 64 milligrams per day) was even greater than the increase found for the 9-gram intake. Takaguchi and coworkers gave their three groups of ten subjects 1 and 2 grams of ascorbic acid daily for 90 to 180 days. They found no significant increase in urinary oxalate excretion. The normal oxalate excretion of their subjects on the same standardized diet before taking the ascorbic acid varied from a low of 11 milligrams to a high of 55 milligrams. El-Dakhakhny and El-Sayed fed 4 grams of ascorbic acid to 8 subjects on the same diet whose urinary oxalate excretion before the ascorbic acid varied from 17 to 132 milligrams per day. In one subject there was no change, in two subjects there was a decrease of 32 to 56 milligrams, while in the other five, increases of 10 to 18 milligrams a day were reported. Evidently there are many other factors beside ascorbic acid that determine urinary oxalate.
While cystine stones are relatively rare, the megascoric approach to their control has never been considered before. Cystine is the insoluble, oxidized form of the acid-soluble, sulfur amino acid cysteine. The soluble form (cysteine) is a stone reducing agent, like ascorbic acid, and both cysteine and ascorbic acid are members of normal biological oxidation-reduction systems. They both interact and protect each other from the bad effects of oxidation. Maintenance of high levels of the highly reducing ascorbic acid in the urinary system of cystinurics (those people prone to cystine stones) may make it possible to maintain their abnormally high cysteine levels in the reduced soluble form and thus avoid conversion to the insoluble cystine, with subsequent crystallization and stone formation. In this way, the excess cysteine could be disposed of in a reduced soluble form in the ascorbic acid-acidified urine. This is similar to the rationale proposed by Schlegel, Pipkin and coworkers (1) who utilized the antioxidant effect of ascorbic acid in the prevention of bladder cancer. This rationale for cystine stone prevention is an entirely new area of clinical research resulting from these megascorbic concepts.