"THIS MAY BE THE MOST IMPORTANT BOOK
ON HEALTH EVER WRITTEN"
- National Health Federation Bulletin -
THE
HEALING FACTOR
VITAMIN C
Against Disease
____________________________
By Irwin Stone
With forewords by Nobel Prizewinners
Dr. Linus Pauling
and
Dr. Albert Szent-Gyorgyi
___________________________________________________
Vitamin C may save your life! A noted biochemist reveals for laymen the exciting research into ascorbic acid's powers against such deadly enemies as cancer, heart disease, strokes, mental illness, old age, diabetes, arthritis, kidney disease, hepatitis -- even cigarette smoking!
AGING - ALLERGIES
ASTHMA - ARTHRITIS - CANCER
COLDS - DIABETES & HYPOGLYCEMIA
EYE TROUBLE - HEART DISEASE - STROKES
KIDNEY & BLADDER AILMENTS - MENTAL ILLNESS
STRESS SYNDROMES - POISONING - POLLUTION
ULCERS - VIRUSES
WOUNDS & FRACTURES
SHOCK
COULD THEY BE
THE RESULT OF VITAMIN C DEFICIENCY?
COULD THEY BE
PREVENTED BY TAKING MORE VITAMIN C?
COULD THEY BE TREATED WITH VITAMIN C?
IS VITAMIN C REALLY A VITAMIN?
IRWIN STONE SAYS YES, YES, YES AND NO!
After 40 years research, Irwin Stone unfolds his startling conclusion that an ancient genetic mutation has left the primate virtually alone among animals in not producing ascorbic acid (Vitamin C) in his own body. By treating it as a "minimum daily requirement" instead of the crucial enzyme it really is, we are living in a state of sub-clinical scurvy whose symptoms have been attributed to other ailments. The answer is to change our thinking about Vitamin C and consume enough to replenish this long-lost "healing factor." Stone illustrates, with massive documentation, Vitamin C's remarkable ability to fight disease, counteract the ill effects of pollution and prolong healthy life -- easily and inexpensively!
GD/Perigee Books
are published by
The Putnam Publishing Group
ISBN 0-399-50764-7
THE
HEALING FACTOR
______________________
"VITAMIN C"
Against Disease
Irwin Stone
A GD/Perigee Book
Perigee Books
are published by
The Putnam Publishing Group
200 Madison Avenue
New York, New York 10016
Copyright © 1972 by Irwin Stone
All rights reserved. This book, or parts thereof,
may not be reproduced in any form without permission.
Published simultaneously in Canada by General Publishing
Co. Limited, Toronto.
Library of Congress Catalog Number: 72-77105
ISBN 0-399-50764-7
First Perigee printing, 1982
Printed in the United States of America
This book is dedicated to my wife, Barbara whose patience and collaboration over the years made it possible.
CONTENTS
Forewords
Linus Pauling
Albert Szent-Gyorgyi
Acknowledgements
Introduction 1
Part I: Our Deadly Inheritance
1. The Beginnings of Life
2. From Fishes to Mammals
3. Our Ancestral Primate
4. The Evolution of Man
5. From Prehistory to the Eighteenth Century
6. The Nineteenth and Early Twentieth Centuries
7. Finding the Elusive Molecule
8. The Genetic Approach
9. Some Effects of Ascorbic Acid
10. "Correcting" Nature
Part II: Pathways to Research
11. Breaking the "Vitamin" Barrier
12. The Common Cold
13. Viral Infection
14. Bacterial Infection
15. Cancer
16. The Heart, Vascular System, and Strokes
17. Arthritis and Rheumatism
18. Aging
19. Allergies, Asthma, and Hay Fever
20. Eye Conditions
21. Ulcers
22. Kidneys and Bladder
23. Diabetes and Hypoglycemia
24. Chemical Stresses -- Poisons, Toxins
25. Physical Stresses
26. Pollution and Smoker's Scurvy
27. Wounds, Bone Fractures, and Shock
28. Pregnancy
29. Mental Disease
30. The Future
References Cited from the Medical Literature
Glossary
The numerals set off in parenthesis in the text are intended to guide the reader to the appropriate medical citation listed at the end of the book.
FOREWORD
by Linus Pauling, Ph.D.
Nobel Laureate
This is an important book -- important to laymen, and important to physicians and scientists interested in the health of people.
Irwin Stone deserves much credit for having marshalled the arguments that indicate that most human beings have been receiving amounts of ascorbic acid less than those required to put them in the best of health. It is his contention, and it is supported by much evidence, that most people in the world have a disease involving a deficient intake of ascorbic acid, a disease that he has named hypoascorbemia. This disease seems to be present because of an evolutionary accident that occurred many millions of years ago. Ancestors of human beings (and of their close present-day relatives, other primates) were living in an area where the natural foods available provided very large amounts of ascorbic acid (very large in comparison with the amounts usually ingested now and the amounts usually recommended now by physicians and other authorities on nutrition). A mutation occurred that removed from the mutant the ability to manufacture ascorbic acid within his own body. Circumstances were such that the mutant had an evolutionary advantage over the other members of the population, who were burdened with the machinery for manufacturing additional ascorbic acid. The result was that the part of the population burdened with this machinery gradually died out, leaving the mutants, who depended upon their food for an adequate supply of ascorbic acid.
As man has spread over the earth and increased in number, the supplies of ascorbic acid have decreased. It is possible that most people in the world receive only one or two percent of the amounts of ascorbic acid that would keep them in the best of health. The resulting hypoascorbemia may be responsible for many of the illnesses that plague mankind.
In this book, Irwin Stone summarizes the evidence. The publication of Irwin Stone's papers and of this book may ultimately result in a great improvement in the health of human beings everywhere, and a great decrease in the amount of suffering caused by disease.
- Linus Pauling -
FOREWORD
by Albert Szent-Gyorgyi, M.D., Ph.D.
Nobel Laureate
My own interest in ascorbic acid centered around its role in vegetable respiration and defense mechanisms. All the same, I always had the feeling that not enough use was made of it for supporting human health. The reasons were rather complex. The medical profession itself took a very narrow and wrong view. Lack of ascorbic acid caused scurvy, so if there was no scurvy there was no lack of ascorbic acid. Nothing could be clearer than this. The only trouble was that scurvy is not a first symptom of lack but a final collapse, a premortal syndrome, and there is a very wide gap between scurvy and full health. But nobody knows what full health is! This could be found out by wide statistical studies, but there is no organization which could and would arrange such studies. Our society spends billions or trillions on killing and destruction but lacks the relatively modest means demanded to keep its own health and prime interest cared for. Full health, in my opinion, is the condition in which we feel best and show the greatest resistance to disease. This leads us into statistics which demand organization. But there is another, more individual difficulty. If you do not have sufficient vitamins and get a cold, and as a sequence pneumonia, your diagnosis will not be "lack of ascorbic acid" but "pneumonia." So you are waylaid immediately.
I think that mankind owes a serious thanks to Irwin Stone for having kept the problem alive and having called Linus Pauling's attention to it.
On my last visit to Sweden, I was told that the final evidence has been found that ascorbic acid is quite harmless. An insane person had the fixed idea that he needed ascorbic acid so he swallowed incredible amounts of it for a considerable period without ill effects. So, apart from very specific conditions, ascorbic acid cannot hurt you. It does not hurt your pocket either, since it is very cheap. It is used for spraying trees.
I also fully agree with Dr. Pauling's contention that individual needs for vitamin C vary within wide limits. Some may need high doses, others may be able to get along with less, but the trouble is that you do not know to which group you belong. The symptoms of lack may be very different. I remember my correspondence with a teacher in my earlier days who told me that he had an antisocial boy whom he was unable to deal with. He gave him ascorbic acid and the boy became one of his most easygoing, obedient pupils. Nor does wealth and rich food necessarily protect against lack of vitamins. I remember my contact with one of the wealthiest royal families of Europe where the young prince had constant temperature and had poor health. On administering vitamin C, the condition readily cleared up.
It gives me great satisfaction to see this book appear and I hope very much that its message will be understood.
- Albert Szent-Gyorgyi -
ACKNOWLEDGMENTS
This book took many years to write and involved many people. Because of a nonexistent budget and the fact that much of the data was in foreign languages, good friends had to be relied upon to supply translations. Among these friends were Lotte and George Bernard, Helene Gottlieb, Dorothy Kramer, Irving Minton, Jutta Nigrin, Sal Scaturo, Tanya Ronger, and Natasha and Otmar Silberstein.
Invaluable help and advice on library work were supplied by Eliphal Streeter and Vera Mitchell Throckmorton. The medical library of the Statin Island Public Health Hospital and the reprint facilities of the National Library of Medicine and the Medical Research Library of Brooklyn were especially helpful.
In any radically new scientific concept, encouragement nd inspiration to carry on are difficult to come by. The author was fortunate in having men of scientific or medical stature such as Linus Pauling, Albert Szent-Gyorgyi, Frederick R. Klenner, Abram Hoffer, William J. McCormick, Thomas A. Garrett, Walter A. Schnyder, Louis A. Wolfe, Alexander F. Knoll, Marvin D. Steinberg, Benjamin Kramer, and A. Herbert Mintz as pillars of strength. Miriam T. Malakoff and Martin Norris supplied editorial advice and encouragement. My wife Barbara, in the latter years, handled the bulk of the library research. To all these people and to many others who have contributed, go my deep gratitude and thanks. I trust that their efforts effectively contribute to better health for man.
Discovery consists in seeing what everybody else has seen and thinking what nobody has thought.
Albert Szent-Gyorgyi
INTRODUCTION
The purpose of this book is to correct an error in orientation which occurred in 1912, when ascorbic acid, twenty years before its actual discovery and synthesis, was designated as the trace nutrient, vitamin C. Thus, in the discussions in this book the terms "vitamin C" and "ascorbic acid" are identical, although the author prefers to use "ascorbic acid."
Scurvy, in 1912, was considered solely as a dietary disturbance. This hypothesis has been accepted practically unchallenged and has dominated scientific and medical thinking for the past sixty years. The purpose of this vitamin C hypothesis was to produce a rationale for the conquest of frank clinical scurvy. That it did and with much success, using minute doses of vitamin C. Frank clinical scurvy is now a rare disease in the developed countries because the amounts of ascorbic acid in certain foodstuffs are sufficient for its prevention. However, in the elimination of frank clinical scurvy, a more insidious condition, subclinical scurvy, remained; since it was less dramatic, it was glossed over and overlooked. Correction of subclinical scurvy needs more ascorbic acid than occurs naturally in our diet, requiring other non-dietary intakes. Subclinical scurvy is the basis for many of the ills of mankind.
Because of this uncritical acceptance of a misaligned nutritional hypothesis, the bulk of the clinical research on the use of ascorbic acid in the treatment of diseases other than scurvy has been more like exercises in home economics than in the therapy of the sequelae of a fatal, genetic liver-enzyme disease. One of the objects of this book is to take the human physiology of ascorbic acid out of the dead-end of nutrition and put it where it belongs, in medical genetics. In medical genetics, wide vistas of preventive medicine and therapy are opened up by the full correction of this human error of carbohydrate metabolism.
For the past sixty years a vast amount of medical data has been collected relating to the use of ascorbic acid in diseases other than scurvy, but only very little practical therapeutic information has developed pertaining to its successful use in these diseases. The reader may well ask what is the difference between data and information? This can be illustrated by the following example: the number 382,436 is just plain data, but 38-24-36, that is information.
The most probable reason for the paucity of definitive therapeutic ascorbic acid information in the therapy of diseases other than scurvy is related to the fact that the vitamin C-oriented investigators were trying to relieve a trace-vitamin dietary disturbance and never used doses large enough to be pharmacologically and therapeutically effective. The new genetic concepts currently correct this old, but now obvious, mistake by supplying a logical rationale for these larger, pharmacologically effective treatments.
If the research suggestions contained in this book are properly and conscientiously followed through, it is the hope of the author that future medical historians may consider this as a major breakthrough in medicine of the latter quarter of the twentieth century.
While many scientific and medical papers have appeared, the publication of Dr. Linus Pauling's book, Vitamin C and the Common Cold, in late 1970 was the first scientific book ever published in the new medical fields of megascorbic prophylaxis and megascorbic therapy, which are branches of orthomolecular medicine. Dr. Pauling's book paved the way for this volume.
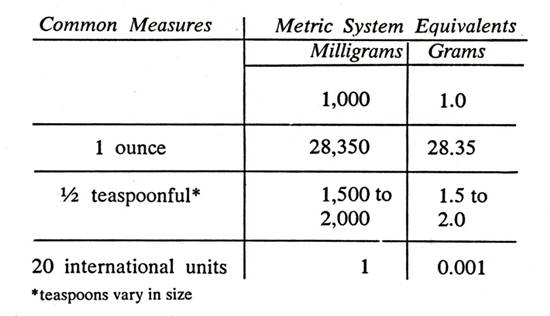
Since the size of the daily intake of ascorbic acid is so important in the later discussions, the reader can refer to the following table of equivalents. The dosages are usually expressed in the metric system in milligrams or grams of ascorbic acid:
PART I
____________________________________________________________
Our Deadly Inheritance
1
____________________________________________________________
The first part of this book is a scientific detective story. The corpus delicti is a chemical molecule, and to collect the evidence in this case we have to cover billions of years in time and have to search in such odd places as frog kidneys, goat livers, and "cabbages and kings." The search will be rewarding because it will contribute to the understanding of this tremendously important molecule. The evidence we unearth will show that the lack of this molecule in humans has contributed to more deaths, sickness, and just plain misery than any other single factor in man's long history. when the molecule is finally discovered and assigned its rightful place in the scheme of things, and its potentialities for good are fully realized, undreamed-of vistas of exuberant health, freedom from disease, and long life will be opened up.
To start on the first leg of our journey, we will have to get into our Time Machine, set the dials, and go back 2.5 to 3 billion years. It will be necessary to seal ourselves completely in the Time Machine and carry a plentiful supply of oxygen because the atmosphere in those days was very different from what it is now. It will be hot and steamy, with little or no oxygen, and besides much water vapor, will contain notable quantities of gases such as carbon dioxide, methane, and ammonia. The hot seas will contain the products of the chemical experiments that Nature had been conducting for millions of years. If we are fortunate, we will arrive on the scene just as Nature was preparing to launch one of its most complicated and organized experiments -- the production of living matter. If we were to sample the hot sea and examine it with our most powerful electron microscope, we would find in this thin consomme' the culmination of these timeless chemical experiments in the form of a macromolecule having the property of being able to make exact duplicates of itself. The term "macromolecule" merely means a huge molecule which is formed out of a conglomeration of smaller unit molecules. The process of forming these huge molecules from the smaller units is called "polymerization," and is similar to building a brick wall (the macromolecule) from bricks (the smaller unit molecules). The "cement" holding the unit molecules together consists of various chemical and physical attractive forces of varying degrees of tenacity.
This self-reproducing macromolecule in this primordial soup might resemble some of our present-day viruses, but it had many important biochemical and biophysical problems to solve before it would begin to resemble some of the more primitive forms of life, such as bacteria, as we know them today. Nature had plenty of time to experiment and eventually came up with successful solutions to problems like heredity, enzyme formation, energy conservation, a protective covering for these naked macromolecules, and then cellular and multicellular organisms. The problem of heredity was solved so successfully by these early self-duplicating macromolecules that our present basis of heredity, the macromolecule DNA, is probably little changed from its original primordial form.
Enzyme formation was a problem that required an early solution if life was to continue evolving, since enzymes are the very foundation of the life process. An enzyme is a substance produced by a living organism which speeds up a specific chemical reaction. A chemical transformation that would require years to complete can be performed in moments by the mere presence of an enzyme. Enzymes are utilized by all living organisms to digest food, transform energy, synthesize tissues, and conduct nearly every biochemical reaction in the life process. The body contains thousands of enzymes.
Energy conservation and utilization was neatly solved in some of these early life forms by the development of photosynthesis: an enzymatic process which uses the energy of sunlight to transform carbon dioxide and water into carbohydrates. Carbohydrates are used for food and structural purposes, and these primitive forms evolved into the vast species of the plant kingdom.
At some time early in the development of life, certain primitive organisms developed the enzymes needed to manufacture a unique substance that offered many solutions to the multiple biological problems of survival. This compound, ascorbic acid, is a relatively simple one compared to the many other huge, complicated molecules produced by living organisms. Because of its unique properties, however, it is somewhat unstable and transient, a fact that will complicate our later search for this substance.
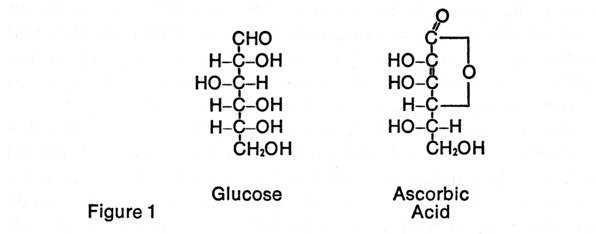
We now know that ascorbic acid is a
carbohydrate derivative containing six carbon atoms, six oxygen atoms, and
eight hydrogen atoms and is closely related to the sugar, glucose (see Figure
1). Glucose is of almost universal occurrence in living organisms, where
it is used as a prime source of energy. Ascorbic acid is produced
enzymatically from this sugar in both plants and animals.
We can surmise that the production of ascorbic acid was an early accomplishment of the life process because of its wide distribution in nearly all present-day living organisms. It is produced in comparatively large amounts in the simplest plants and the most complex; it is synthesized in the most primitive animal species as well as in the most highly organized. Except possibly for a few microorganisms, those species of animals that cannot make their own ascorbic acid are the exceptions and require it in their food if they are to survive. Without it, life cannot exist. Because of its nearly universal presence in both plants and animals we can also assume that its production was well organized before the time when evolving life forms diverged along separate plant and animal lines.
This early development of the ascorbic acid synthesizing mechanisms probably arose from the need of these primitive living organisms to capture electrons from an environment with very low levels of oxygen. This process of scavenging for rare oxygen was a great advance for the survival and development of the organisms so equipped. It also may have triggered the development of the photo-synthetic process and sparked the tremendous development of plant life. This great increase in plant life, with its use of the energy of sunlight to produce oxygen and remove carbon dioxide from the atmosphere, completely changed the chemical composition of the atmosphere, over a period of possibly a billion years, from oxygen-free air which would not support living animals a we know them to a life-giving oxygen supply approaching the composition of our present atmosphere.
The increase in the oxygen content of the atmosphere had other important consequences. In the upper reaches of the atmosphere, oxygen is changed by radiation into ozone, which is a more active form of oxygen. This layer of high-altitude ozone acts as a filter to remove the deadly ultraviolet rays from sunlight and makes life on land possible. This series of events, which occurred more than 600 million years ago, preceded the tremendous forward surge of life and the development of more complicated, multicellular organisms in post-Cambrian times, as is seen in the fossil record.
The only living organisms that survive to this day in a form that has not progressed or evolved much from the forms which existed in the earliest infra-Cambrian times are primitive single-cell organisms, such as bacteria, which do not make (and may not need ascorbic acid in their living environment. All plants or animals which have evolved into complex multicellular forms make or need ascorbic acid. Was ascorbic acid the stimulus for the evolution of multicellular organisms? if not the stimulus, it certainly increased the biochemical adaptability necessary for survival in changing and unfavorable environments.
Further evidence for the great antiquity of the ascorbic acid-synthesizing systems may be obtained from the science of embryology. During its rapid fetal development, the embryo passes through the various evolutionary stages that its species went through in time. This led nineteenth-century embryologists to coin the phrase, "ontogeny recapitulates phylogeny," which is another way of saying the same thing. In fetal development, ascorbic acid can be detected very early, when the embryo is nothing more than a shapeless mass of cells. For instance, in the development of the chick embryo (which is convenient to work with), the chicken egg is devoid of ascorbic acid, but it can be detected in the early blastoderm stage of the growing embryo. At this stage the embryo is just a mass of cells in which no definite organs have as yet appeared, and it resembles the most primitive multicellular organisms -- both fossil and present living forms. In plants, also, the seeds have no ascorbic acid, but a soon as the plant embryo start to develop, ascorbic acid is immediately formed. Thus all the available evidence points to the great antiquity of the ascorbic acid-synthesizing systems in life on this planet.
2
_____________________________________________________________
If we reset the dials in our Time Machine and travel to a point about 450 million years ago, we may be able to witness the start of another notable experiment by Nature. In the seas are the beginnings of the vertebrates, a long line of animals that will eventually evolve into the mammals and man. These are the animals with a more or less rigid backbone, containing the start of a well-organized and complex nervous and muscular system, and capable of reacting much more efficiently to their environment than the swarms of simpler, spineless invertebrates, which had apparently reached the end of their evolutionary rope. Nature was ready to embark on another revolutionary, and more complicated, experiment.
Because of the increased complexity of their nervous system and a fast-acting muscular system, these primitive vertebrate fishes were able to gather food better and avoid enemies and other perils, all of which had increased survival value. Before they could do this, however, they had to develop complex, specialized organ systems in which various biochemical processes were carried out. And their requirements for ascorbic acid were undoubtedly much higher because of their much increased activity. The simpler structures of the invertebrates no longer sufficed and required much modification to suit the needs of these more active and alert upstarts, the vertebrates.
The vertebrate fishes were such a successful evolutionary experiment that for the next 100 million years or so they dominated the waters. Nature was now ready to carry out another experiment -- that of taking the animals out of the crowded seas and putting them on dry land. It had experience in this sort of operation since the plants had long ago left the seas and were well established on land. The land was no longer a place of barren fields, but was covered with dense vegetation. Two lines of modification were tried: in one, the fish was structurally modified so that it could clumsily exist out of the water; in the other, a more complete renovation job was done. Modifications of the fins and the swim bladder ended in the evolutionary blind alley of the lung fishes, but the more ambitious program -- involving a complete change in the biochemistry and life cycle -- produced a more successful line -- the amphibians. These creatures are born in the water and spend their early life there and then they metamorphose into land-living forms. Frogs and salamanders are present-day denizens of this group. The next step in evolution was to produce wholly land-living animals -- the reptiles. These were scaly animals that slithered, crawled, walked, or ran; and some grew to prodigious size. Some preferred swimming and reverted to the water and others took to the air. It was these airborne species that eventually evolved into the warm-blooded birds. The birds are of particular interest to us because they solved an ascorbic acid problem in the same fashion as the primitive mammals, which were appearing on the scene at about this time.
We have gone into this cursory sketch of this period of evolutionary history to trace the possible history of ascorbic acid in these ancient animals. If we assume that the present-day representatives of the amphibians, the reptiles, the birds, and the mammals have the same biochemical systems as their remote ancestors, then we can do some more detective work on our elusive molecule. These complex vertebrates all have well-defined organ systems that are assigned certain definite functions. Usually an organ has a main biological function and also many other accessory, but no less important, biochemical responsibilities. The kidney, whose main function is that of selective filtration and excretion, is also the repository of enzyme systems for the production of vitally important chemicals needed by the body. The liver, which is the largest organ of the body, functions mainly to neutralize poisons, produce bile, and act as a storage depot for carbohydrate reserves; but it also has many other duties to perform.
In examining present-day creatures we find that in the fishes, amphibians, and reptiles, the place where ascorbic acid is produced in the body is localized in the kidney. When we investigate the higher vertebrates, the mammals, we find that the liver is the production site and the kidneys are inactive. Apparently, during the course of evolution the production of enzymes for the synthesis of ascorbic acid was shifted from the small, biochemically crowded kidneys to the more ample space of the liver. This shift was the evolutionary response to the needs of the more highly developed species for greater supplies of this vital substance.
The birds are of particular interest because they illustrate this transition. In the older orders of the birds, such as the chickens, pigeons, and owls, the enzymes for synthesizing ascorbic acid are in the kidneys. In the more recently evolved species, such as the mynas and song birds, both the kidneys and the liver are sites of synthesis; and in other species only the liver is active and the kidneys are no longer involved in the manufacture of ascorbic acid. Thus we have a panoramic picture of this evolutionary change in the birds, where the process has been "frozen" in their physiology for millions of years.
This evolutionary shift from the kidneys to the liver took place at a time when temperature regulatory mechanisms were evolving and warm-blooded animals were developing from the previous cold-blooded vertebrates. In the cold-blooded amphibians and reptiles, the amounts of ascorbic acid that were produced in their small kidneys sufficed for their needs. However, as soon as temperature regulatory means were evolved -- producing the highly active, warm-blooded mammals -- the biochemically crowded kidneys could no longer supply ascorbic acid in ample quantities. Both the birds and the mammals, the two concurrently evolving lines of vertebrates, independently arrived at the same solution to their physiological problem: the shift to the liver.
3
_____________________________________________________________
If we come forward to a time some 55 to 65 million years ago we will find that the warm-blooded vertebrates are the dominant animals, and they are getting ready to evolve into forms that are familiar to us. Life has come a long way since it discovered how to make ascorbic acid. In the warmer areas the vegetation is dense and the ancestors of our present-day primates -- the monkeys,apes, and man -- shared the forests and treetops with the innumerable birds.
At about this time something very serious happened to a common ancestor of ours, the animal who would be a progenitor of some of the present primates. This animal suffered a mutation that eliminated an important enzyme from its biochemical makeup. The lack of this enzyme could have proved deadly to the species and we would not be here to read about it except for a fortuitous combination of circumstances.
Perhaps we should digress here and review some facts of mammalian biochemistry as related to this potentially lethal genetic accident. It will not be difficult, and it will help in understanding the thesis of this book.
All familiar animals are built from billions of cells. Masses of cells form the different tissues, the tissues form organs, and the whole animal is a collection of organs. The cell is the ultimate unit of life. Each cell has a cell membrane, which separates it from neighboring cells and encloses a jellylike mass of living stuff. Floating in this living matter is the nucleus, which is something like another, smaller cell within the cell. This nucleus contains the reproducing macromolecule called desoxyribonucleic acid (DNA). DNA is the biochemical basis of heredity and determines whether the growing cells will develop into an oak tree, a fish, a man or whatever. This molecule is a long, thin, double-stranded spiral containing linear sequences of four different basic unit molecules. The sequence of the four unit molecules as they are arranged on this spiral is the code that forms the hereditary blueprint of the organism. When a cell divides, this double-stranded molecule separates into two single strands and each daughter cell receives one. in the daughter cell, the single strand reproduces an exact copy of itself to again form the double strand and in this way each cell contains a copy of the hereditary pattern of the organism.
These long threadlike molecules are coiled and form bodies in the nucleus that were called chromosomes by early microscopists because they avidly absorbed dyes and stains and thus became readily visible in preparations viewed microscopically. These microscopitst suspected that these bodies were in some way connected with the process of inheritance but did not know the exact mechanisms.
Certain limited sections of these long, spiral molecules, which direct or control a single property such as the synthesis of a single enzyme, are called genes. A chromosome may be made up of thousands of genes. The exact order of the four different unit molecules in a gene determines, say, the protein structure of an enzyme. If only one of these unit molecules is out of place or transposed among the thousands in a gene sequence, the protein structure of the enzyme will be modified and its enzymatic activity may be changed or destroyed. Such a change in the sequence of a DNA molecule is called a mutation.
These mutations can be produced experimentally by means of various chemicals and by radiations such as X rays, ultraviolet rays, or gamma rays. Cosmic rays, in nature, are not doubt a factor in inducing mutations. It is on these mutations that Nature has depended to produce changes in evolving organisms. If the mutation is favorable and gives the plant or animal an advantage in survival, it is transmitted to its descendants. If it is unfavorable and produces death before reproduction takes place, the mutation dies out with the mutated individual and is regarded as a lethal mutation. Some unfavorable mutations which are serious enough to be lethal, but which the mutated animal survives, are called conditional lethal mutations. This type of mutation struck a primitive monkey that was the ancestor of man and some of our present-day primates.
In nearly all the mammals, ascorbic acid is manufactured in the liver from the blood sugar, glucose. The conversion proceeds stepwise, each step being controlled by a different enzyme. The mutation that occurred in our ancestral monkey destroyed his ability to manufacture the last enzyme in this series -- L-gulonolactone oxidase. This prevented his liver from converting L-gulonolactone into ascorbic acid, which was needed to carry out the various biochemical processes of life. The lack of this enzyme made this animal susceptible to the deadly disease, scurvy. To this day, millions of years later, all the descendants of this mutated animal, including man, have the intermediate enzymes but lack the last one. And that is why man cannot make ascorbic acid in his liver.
This was a serious mutation because organisms without ascorbic acid do not last very long. However, by a fortuitous combination of circumstances, the animal survived. First of all, the mutated animal was living in a tropical or semitropical environment where fresh vegetation, insects, and small animals were available the year round as a food supply. All these are good dietary sources of ascorbic acid. Secondly, the amount of ascorbic acid needed for mere survival is low and could be met from these available sources of food. This is not to say that this animal was getting as much ascorbic acid from its food as it would have produced in its own body if it had not mutated. While the amount may not have been optimal, it was sufficient to ward off death from scurvy. Under these ideal conditions the mutation was not serious enough to have too adverse an effect on survival. It was only later when this animal's descendants moved from these ideal surroundings, this Garden of Eden, and became "civilized" that they -- we -- ran into trouble.
This defective gene has been transmitted for millions of years right up to the present-day primates. This makes man and a few other primates unique among the present-day mammals. Nearly all other mammals manufacture ascorbic acid in their livers in amounts sufficient to satisfy their physiological requirements. This had great survival value for these mammals, who, when subjected to stress, were able to produce much larger amounts of ascorbic acid to counteract adverse biochemical effects. And there was plenty of stress for an animal living in the wild, competing for scarce food, and trying to avoid becoming a choice morsel for some other predator.
To the best of our knowledge, only two other non-primate mammals have suffered a similar mutation and have survived. How many others may have similarly mutated and died off, we shall never know. The guinea pig survived in the warm lush forests of New Guinea where vegetation rich in fresh ascorbic acid was readily available. The other mammal is a fruit-eating bat (Pteropus medius) from India. The only other vertebrates that are known to harbor this defective gene are certain passeriforme birds.
Because of this missing or defective gene, man, some of the other primates, the guinea pig, and a bat will develop and die of scurvy if deprived of an outside source of ascorbic acid. A guinea pig, for example, will die a horrible death within two weeks if totally deprived of ascorbic acid in its diet.
4
_____________________________________________________________
In the last chapter it was estimated that the conditional lethal mutation which destroyed our ancestral monkey's ability to produce his own ascorbic acid occurred some 55 to 60 million years ago. Actually, we do not know which primate ancestor was afflicted with this mutation, nor can we exactly pinpoint this occurrence in the time scale. Up to the time of this writing, little work has been done to try to obtain this important data. However, for the purposes of our discussion, it is not essential to know exactly which of man's ancestors was burdened with this genetic defect nor when it happened. It is sufficient to know that it happened before man came on the scene. Presently available evidence indicates that the members of the primate suborder Anthropoidea, the old world monkeys, the new world monkeys, the apes and man, still carry this defective gene.
While this defect did not wipe out the mutated species, it must have put these animals at a serious disadvantage. Normally, a mammal equipped to synthesize its own ascorbic acid will produce it in variable amounts depending upon the stresses the animal is undergoing. These animals have a feedback mechanism that produces more when the animal needs more. The stresses of living under wild and unfavorable conditions were great. The animals had to continually keep out of the way of predators while they searched for food; they had to procreate and take care of their young; they had to resist such environmental stresses as heat, cold, rain, and snow, and the biological stresses of parasites and diseases of bacterial, fungal, and viral origin. Animals that were able to cope with these stresses by producing optimal amounts of ascorbic acid within their bodies were more resistant to environmental extremes of heat and cold, better able to fight off infections and disease, better able to recover from physical trauma, and better prepared to do it repeatedly. The presence of ascorbic acid-synthesizing enzymes conferred enormous powers of survival.
It is likely that our mutated ancestors never got enough ascorbic acid from their food to completely combat all the biochemical stresses. It is equally certain that the amounts they ingested were enough to ensure their survival until they were able to reproduce and raise their young. Their genetic defect must surely have been a serious handicap in their fight for survival, but they did survive.
If we follow the trunk of the evolving primate tree, we come to animals that we know from their fossil remains. Propliopithecus, Proconsul, Oreopithecus, and Ramapithecus, to name a few, may have been the ancestors of the present-day higher primates. These animals were more ape than man, but we are coming closer. About a million years ago we find Australopithecus of Southern Africa. These creatures were no longer living an arboreal existence, swinging through the branches of the forest; they had come down to earth and lived in the wide-open spaces of the grassy plains. While in appearance they still resembled chimpanzees, they held their heads erect, not thrust forward; and they were built to walk erect most of the time. Their hands had fingers too slender to walk upon and their jaws contained teeth more human-like.
In the last million years, evolution developed recognizably human forms. Heidelberg man, Pithecanthropus, Sinanthropus, Swanscombe man, and others bring us to a point about 100,000 years ago. From here on we find the remains of manlike creatures widely distributed: Neanderthal man in the Near East and Europe; Rhodesian man in South Africa; and later, 20,000 to 40,000 years ago, modern man in Europe, Asia, South Africa, and America. The Neanderthal man of 50,000 to 70,000 years ago was a great hunter and went after the biggest and fiercest animals, the woolly rhinoceroses and mammoths. Appetites and diets evolved from the vegetation, fruits, nuts, and insects of the arboreal monkeys, to the raw red meat of these primitive hunters. Fresh raw meat is a good source of ascorbic acid, and Neanderthal man needed plenty of it to survive the climate that had started to turn cold, and the glaciers that covered most of Europe about 50,000 years ago. From a study of a Neanderthal skeleton found in France about a half century ago, it was concluded that Neanderthal man did not stand erect but walked with knees bent in an uncertain, shuffling gait. However it was later discovered that this skeleton was that of an older man with a severe case of arthritis -- fossil evidence of a pattern which might indicate the lack of sufficient ascorbic acid to overcome the stresses of Ice-Age cold and infection.
The evolution of the nervous system and the explosive development of the brain and intelligence compensated in some measure for this biochemical defect by finding new sources of ascorbic acid. The normally herbivorous and insect-eating species became hunters after raw red meat and fish, and later went on to raise their own animals. It was this change in dietary practice that permitted the wide dispersion of the humanoids. They were no longer limited to tropical or semitropical areas where plants or insects rich in ascorbic acid were available the year round. To gauge the effect of this factor, just compare the wide dispersion of man on the face of this globe with his less adaptable primate relatives, the monkeys and apes. They are still swinging in the branches close to their supply of ascorbic acid.
This dispersal of primitive man into environs for which he was not biochemically adapted was not easy and was only accomplished at a high cost -- increased mortality, shortened life span, and great physical suffering. Man's survival under these unfavorable conditions is a tribute to this guts and brainpower. Here was one of Nature's first experiences with an organism that could fight back against an unfavorable environment and win. But the victory, as we can see all around us, was a conditional one.
A study of the remains found in ancient burial grounds reveals some of the privations suffered by Stone Age man and his descendants. Living in the temperate zone, the cold was a constant threat to his existence. The exhumed bones give evidence of much disease, nutritional deficiencies, and general starvation. Infant and child mortality was enormous, and the life span of those who survived their teens was rarely beyond thirty or thirty-five years. Some abnormalities evidenced in the fossilized limbs of these ancient populations could have been caused by recurring episodes of inadequate intake of ascorbic acid. Future studies in paleopathology should bring additional interesting facts to light.
Another way of assessing the extent of the ascorbic acid nutriture of these ancient peoples is to find out what current primitive societies used as food, and what their methods of food preparation and preservation were before "civilization" reached them: the Australian aborigine, the native tribes of Africa, the Indians of the Americas, and the Eskimo, who has worked out a pattern for survival in a most unfavorable environment.
In reviewing the diets of these peoples, one is impressed by their variety. All products of the plant and animal kingdoms,without exception, were at one time or another consumer. Those who ate their food fresh and raw had more chance of obtaining ascorbic acid. Extended cooking or drying tends to destroy ascorbic acid. The availability of food appears to have been the main factor in an individual's nutrition and, aside from certain tribal taboos, there were no aesthetic qualms against eating any kind of food. A luscious spider's abdomen bitten off and eaten raw, a succulent lightly toasted locust consumed like a barbecued shrimp, the larva of the dung beetle soaked in coconut milk and roasted were good sources of ascorbic acid. similarly, raw fish, seaweed, snails, and every variety of marine mollusk were tasty morsels for those peoples living near the shores.
The name "Eskimo" comes from the Cree Indian work "uskipoo," meaning "he eats raw meat." During the long winter, Eskimos depended upon the ascorbic acid content of raw fish or freshly caught raw seal meat and blood. Any Eskimo who cooked his fish and meat -- thereby reducing its ascorbic acid content -- would never have survived long enough to tell about his new-fangled technique of food preparation. But, not even the best of these diets ever supplied ascorbic acid in the amounts that would have been produced in man's own liver if he had the missing gene. The levels were, as always, greatly submarginal for optimal health and longevity, especially under high-stress conditions. The estimated life expectancy for an Eskimo man in northern Greenland is only twenty-five years.
Two great advances in the early history of man were the development of agriculture and the domestication of animals. In the temperate zones, agriculture tended to concentrate on cereal grains or other seed crops, which could easily be stored without deterioration and used during the long winters. These crops are notably lacking in ascorbic acid and, while they supplied calories, scurvy would soon develop in those who depended on them as a staple diet. Whatever fresh vegetables or fruits were grown were generally rendered useless as an antiscorbutic foodstuff for winter use by the primitive methods of drying and preservation.
A trick for imparting antiscorbutic qualities to seed crops was discovered by various agricultural peoples and then forgotten. It was rediscovered again in Germany in 1912, and it has persisted among some Asiatic people. This simple life-saving measure was to take portions of these seed crops (beans and the like), soak them in water, and then allow them to germinate and sprout. The sprouted seeds were consumed. Ascorbic acid is required by the growing plant and it is one of the first substances that is synthesized in the growing seed to nourish the plant embryo. Bean sprouts are even now a common item of the Chinese cuisine as they have been for thousands of years; but they contain our elusive molecule, while unsprouted beans do not.
The early people whose culture was based upon animal husbandry may have fared better in the winter than the agricultural people. they had a built-in continuous ascorbic acid supply in their fresh milk, fresh meat, and blood. If they used these products fresh they were safe, but if they attempted long preservation, the antiscorbutic properties were lost and the foodstuffs became potential poisons.
All in all, it has been a terrible struggle throughout prehistory and history to obtain the little daily speck of ascorbic acid required for mere survival.
5
_____________________________________________________________
FROM PREHISTORY TO THE EIGHTEENTH CENTURY
Now we come to man in historical times and find that he has been plagued by the effects of this genetic defect from the earliest days of recorded history. Before discussing this great scourge of mankind, let us examine the disease caused by this genetic defect. Clinical scurvy is such a loathsome and fatal affliction that it is difficult to conceive that an amount of ascorbic acid that could be piled upon the head of a pin is enough to prevent its fatal effects.
In describing the disease we must distinguish between chronic scurvy and acute scurvy. Chronic or biochemical scurvy is a disease that practically everyone suffers from and its individual severity depends upon the amount of one's daily intake of ascorbic acid. It is a condition where the normal biochemical processes of the body are not functioning at optimal levels because of the lack of sufficient ascorbic acid. There are all shades of biochemical scurvy and it can vary from a mild "not feeling right" to conditions where one's resistance is greatly lowered and susceptibility to disease, stress, and trauma is increased. The chronic form usually exists without showing the clinical signs of the acute form and this makes it difficult to detect and diagnose without special biochemical testing procedures. The acute form is the "classical" scurvy recognized from ancient times and is due to prolonged deprivation of ascorbic acid, usually combined with severe stress.
The first symptom of acute scurvy in an adult is a change in complexion: the color becomes sallow or muddy. There is a loss of accustomed vigor, increased lassitude, quick tiring, breathlessness, a marked disinclination for exertion, and a desire for sleep. There may be fleeting pains in the joints and limbs, especially the legs. Very soon the gums become sore, bleed readily, and are congested and spongy. Reddish spots (small hemorrhages) appear on the skin, especially on the legs at the sites of hair follicles. Sometimes there are nosebleeds or the eyelids become swollen and purple or the urine contains blood. These signs progress steadily -- the complexion becomes dingy and brownish, the weakness increases, with the slightest exertion causing palpitation and breathlessness. The gums become spongier and bleed, the teeth become loose and may fall out, the jawbone starts to rot,and the breath is extremely foul. Hemorrhages into any part of the body may ensue. Old healed wounds and scars on the body may break open and fresh wounds and sores show no tendency to heal. Pains in the limbs render the victim helpless. The gums swell so much that they overlay and hide the teeth and may protrude from the mouth. The bones become so brittle that a leg may be broken by merely moving it in bed. The joints become so disorganized that a rattling noise can be heard from the bones grating against each other when the patient is moved. Death usually comes rapidly from sudden collapse at slight exertion or from a secondary infection, such as pneumonia. This sequence of events, from apparent health to death, may take only a few months.
Acute clinical scurvy was recognized early by
ancient physicians and was probably known long before the dawn of recorded
history. Each year in the colder climes, as winter closed in, the
populations were forced onto a diet of cereal grains and dried or salted meat
or fish, all low in ascorbic acid. Foods rich in ascorbic acid were
scarce if not entirely lacking. The consequence of this inadequate diet
was that near the end of winter and in early spring, whole populations were
becoming increasingly scorbutic. Thus weakened, their resistance low,
people were easy prey for the rampant bacterial and viral infections that
decimated the population. This happened year after year for centuries and
this is the origin of the so-called "spring tonics," which were
attempts to alleviate this annually recurring scurvy (by measures which were
generally ineffective). The number of lives lost in this annual debacle
and the toll in human misery are inestimable. People became so accustomed to
this recurring catastrophe that it was looked upon as the normal course of
events and casually accepted as such. Only in times of civil strife, of
wars and sieges, or on long voyages, where the toll in lives lost to this dread
disease was so great, did it merit special notice.
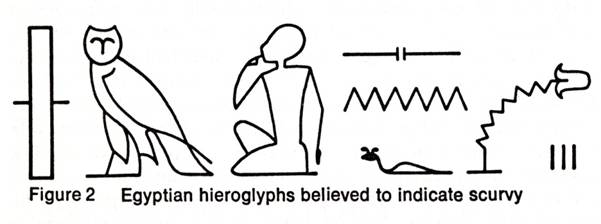
The earliest written reference to a condition that is recognizable as scurvy is in the Ebers papyrus, a record of Egyptian medical lore written about 1500 B.C. Figure 2 shows various Egyptian hieroglyphs for scurvy. The figure of the little man pointing to his mouth and the lips oozing blood indicated the bleeding gums of the disease. It is likely that scurvy was clearly recognized at least 3,000 years ago. Hippocrates (ca. 400 B.C.), the father of medicine, described diseases that sounded suspiciously like scurvy. Pliny the Elder (A.D. 23-79), in his Natural History, describes a disease of Roman soldiers in Germany whose symptoms bespeak of scurvy and which was cured by a plant herba Britannica. Sire Jean de Joinville, in his history of the invasion of Egypt by the Crusaders of Saint Louis in 1260, gives a detailed description of the scurvy that afflicted this army. He mentions the hemorrhagic spots, the fungous and putrid gums, and the legs being affected. It was scurvy that led to the ultimate defeat and capture of Saint Louis and his knights. It is certain that, throughout the Crusades, scurvy took a far greater toll of the Crusaders than all the weapons of the Saracens.
In the great cycle of epidemics that hit Europe in the fourteenth century -- the Black Death of the Middle Ages -- millions of people died. The Black Death was a fulminating, virulent epidemic of a bacterial disease, bubonic plague, concurrent with pulmonary infection superimposed on scurvy with its diffused superficial hemorrhages that caused the skin to turn black or bluish-black. The fact that the disease attacked a population that was first thoroughly weakened by scurvy accounts for the extremely high mortality: one-fourth of the population of Europe -- or 25 million deaths. it is known that resistance to infection is lowered by a deficiency of ascorbic acid, so a disease which would normally be a mild affliction could whip through a scorbutic population with unprecedented fierceness and fatally strike down thousands.
With the invention of the printing press and the easier dissemination of the printed word, the sixteenth and following centuries saw the appearance of many tracts that described scurvy and its bizarre causes, and offered many different exotic treatments and "cures" for the disease. Much earlier, folklore had associated scurvy with a lack of fresh foodstuffs, and the antiscorbutic qualities of many plants had been known. But these qualities were forgotten and had to be rediscovered again and again at a great cost in lives and suffering.
Improved ship construction and the ensuing long voyages provided ideal conditions for the rapid development of acute scurvy under circumstances where the developing symptoms could be readily observed and recorded. Sailors quickly succumbed to scurvy due to inadequate diet; physical exertions; exposure to extremes of heat, cold, and dampness; and the generally unsanitary shipboard conditions. In a few short months, out of what started off as a seemingly healthy crew, only a few remained fit for duty and were able to stand watch. The logs of these voyages make incredible reading today. Before scurvy was finally controlled, this scourge destroyed more sailors than all other causes, including the extremely high tolls of naval warfare.
In 1497, Vasco da Gama, while attempting to find a passage to the Indies by way of the Cape of Good Hope, lost 100 of his 160-man crew to scurvy. Magellan, in 1519, set sail with a fleet of five ships on one of history's great voyages, the circumnavigation of the earth. Three years later, only one ship, with only eighteen members of the original crew, returned to Spain. On occasion, a Spanish galleon would be found drifting, a derelict, its entire crew dead of scurvy. Many books were written on scurvy during the sixteenth to eighteenth centuries; some authors hit upon means to actually combat the disease, while others, clouded by the medical lore of the times, were way off base.
We still find logs of eighteenth-century voyages that recount the devastating effects of scurvy, and others where the master of the ship was able to prevent the disease. In 1740, Commodore Anson left England with six vessels and 1500 seamen; he returned four years later with one ship and 335 men. Between 1772 and 1775, on his round-the-world trip, however, Captain James Cook lost only one man out of his crew of 118, and that one not from scurvy. Cook took every opportunity when touching land to obtain supplies of fresh vegetables and fruit. He usually had a good store of sauerkraut aboard and he knew the beneficial qualities of celery and scurvy grass. After the voyage was completed, Cook was presented with the Copley Medal of the Royal Society. This award was given for his success in making such a lengthy voyage without a single death from scurvy -- not for his great navigational and geographic discoveries. His scientific contemporaries understood the great significance of Cook's accomplishment. Between the time of Anson's failure and Cook's great success another important event took place; the first modern type of medical experiment was carried out by James Lind. We will discuss this later.
This is only a very brief record of the easily avoidable havoc caused by scurvy on the high seas, but those on land fared little better. In addition to the fearful, annually recurring scorbutic devastation of the population in the late winter and early spring, there were special circumstances which brought on deadly epidemics of acute scurvy. Wars and long sieges brought these epidemics to a head. In a brief sampling of the wars of the sixteenth to eighteenth century, scurvy appeared at the siege of Breda in Holland in 1625 and at Thorn in Prussia in 1703, where it accounted for 5,000 deaths among the garrison and noncombatants. It took its toll of the Russian armies in 1720 in the war between the Austrians and the Turks, of the English troops that captured Quebec in 1759, and of the French soldiers in the Alps in the spring of 1795.
Witness also Jaques Cartier's expedition to Newfoundland in 1535. These were men from a civilized culture with a long background of medical lore. One hundred of Cartier's 110 men were dying of scurvy until the Indian natives showed him how to make a decoction from the tips of spruce fir which cured his men. This trick, incidentally, was also used by the defenders of Stalingrad in World War II to stave off scurvy.
By the middle of the eighteenth century ,the stage had been prepared for advances in the prevention and treatment of scurvy. Admiral Sir Richard Hawkins, in 1593, protected the crew of the Dainty with oranges and lemons; among others, Commodore Lancaster, in voyages for the East India Company, had shown by 1600 that scurvy was an easily preventable disease. It remained, however, for James Lind to prove this. James Lind, surgeon's mate on H.M.S. Salisbury, was inspired by the hardships of the Anson fiasco and the many cases of scurvy he had treated on his own ships. Lind was a keen observer and eventually became known as "the father of nautical medicine." He conducted the first properly controlled clinical therapeutic trial on record.
The crucial experiment Lind performed in 1747 at sea on the Salisbury, was to take twelve seamen suffering from the same degree of scurvy and divide them into six groups of two each. In addition to their regular diet, he gave each group a different, commonly used treatment for scurvy and observed its action. One group received a quart of cider daily, the second group received twenty-five drops of dilute sulfuric acid three times a day, the third group was given two spoonfuls of vinegar three times a day, the fourth team drank half a pint of seawater three times a day, the fifth received a concoction of garlic, mustard seed, horseradish, gum myrrh, and balsam of Peru. The last group received two oranges and one lemon daily for six days. These last two men improved with such astonishing rapidity that they were used as nurses to care for the others. There was slight improvement in the cider group but no benefit was observed in the others. Here was clear-cut, easily understandable evidence of the value of citrus fruit in the cure of scurvy. Although Lind did not realize it, he had found a good natural source of our elusive molecule.
Lind left the Royal Navy in 1748, obtained an M.D. degree from Edinburgh University, and went into private practice. He was later physician at the Royal Naval Hospital at Haslar and physician to the Royal Household of George III at Windsor. He continued his work on scurvy and, in 1753, published one of the classics of medical literature, A Treatise of the Scurvy. What as clear to Lind, and is commonplace to us, was not so readily accepted by the naval bureaucracy of his day. It took over forty years for the British Admiralty to adopt Lind's simple prophylactic daily dose of one ounce of lemon juice per man. The official order cam through in 1795, just a year after Lind's death. It has been estimated that this 42-year delay cost the Royal Navy 100,000 casualties to scurvy.
This simple regimen wiped out scurvy in the naval forces of England, and it became their secret weapon for maintaining their mastery of the seas. There is no doubt that this simple ration of lemon juice was of far greater importance to the Royal Navy of the eighteenth and nineteenth centuries than all the improvements in speed, firepower, armor, and seaworthiness. Naval officers of the time asserted that it was equivalent to doubling the fighting force of the navy. Previously, because of the ravages of scurvy, the seagoing fleets had to be relieved every ten weeks by a freshly manned fleet of equal strength so that the scorbutic seamen could be brought home for rehabilitation. The impact of our elusive molecule on history has never been adequately evaluated. In this case Lind did as much as Nelson to break the power of Napoleon. The English vessels were able to maintain continuous blockade duties, laying off the coast of France for months at a time without the necessity of relieving the men. Were it not for Lind, the flat-bottomed invasion barges assembled by Napoleon may well have crossed the English Channel.
6
_____________________________________________________________
EARLY TWENTIETH CENTURIES
One would imagine that Lind's clear-cut clinical demonstration and the experiences of the Royal Navy in wiping out the disease would have pointed the way toward banishing this disease completely. However, it takes much more than logic and clear-cut demonstrations to overcome the inertia and dogma of established thought. The forty-two years that it took the British Admiralty to adopt Lind's recommendations may seem unduly long, even for a stolid bureaucracy, but this may be a speed record compared with other agencies. The British Board of Trade took 112 years -- until 1865 -- before similar precautions were adopted for the British merchant marine. There are records of seamen on the merchant vessels succumbing to scurvy even while delivering lemons to the ships of the Royal Navy. Over 30,000 cases of scurvy were reported in the American Civil War and it took the U.S. Army until 1895 to adopt antiscorbutic rations.
The saga of scurvy continues, with its incredible toll in human lives and suffering, up to the present day. In the nineteenth century, 104 land epidemics have been tabulated. And the twentieth century has had plenty of trouble with the disease, not only as a result of the World Wars but in civil populations, as a result of ignorance and improper use of food.
In the latter part of the nineteenth century Barlow's disease became prevalent, and it was recognized as infantile scurvy. It appeared at the time when artificial feeding of babies was becoming popular, and took the name of the doctor who described the disease in 1883. Actually, the disease was first described in 1650, but it was confused with rickets until Barlow's clear differentiation. There were so many cases that it was also known as Mueller's or Cheadle's disease. Breast-fed babies did not appear to get the disease, but those fed with boiled and heated cow's milk or with cereal substitutes did. It is an intensively painful disease and results in stunted growth and delayed development. The disease persists to the present day and is amenable to the same preventive and curative measures as adult scurvy -- the speck of ascorbic acid contained in fresh fruits and vegetables. Here again the lesson had to be relearned, the hard way, with babies put in the same class as seamen.
Scurvy and its treatment has had many ups and downs during the long history of man, and one series of events in the nineteenth century raised some mistrust in the prophylactic powers of fresh fruits. This is another example of the kind of mistaken conclusions based on confusion, incomplete observations, and improper interpretations which has cursed the history of scurvy. At the time this took place all knowledge of scurvy was completely empirical; there was no experimental or quantitative data because there were no experimental animals known that could be given scurvy. We did not know any more than Lind knew a hundred years before.
The English used the term "lime" indiscriminately for both lemons and limes. In 1850, for political and economic reasons they substituted the West Indian lime for the traditional Mediterranean lemon used by the Royal Navy since 1795. We now know that the Mediterranean lemon is a good source of our elusive molecule, while the West Indian lime is not. In 1875 the Admiralty supplied a large amount of West Indian lime juice to Sir George Nare's expedition to discover the North Pole. An epidemic of scurvy broke out and ruined the expedition. A commission was appointed to inquire into the cause of the disaster but could arrive at no satisfactory conclusion. It was even more perplexing because a previous Arctic expedition in 1850 (the date is important because lemons were used) had spent two years of great hardships, but without scurvy. It took until 1919 to finally resolve the cause of this debacle; in the meantime, however, this incident provoked a general and indiscriminate distrust of antiscorbutics -- especially among Polar explorers. On the Jackson-Harmsworth expedition of 1894-1897, a party carrying no lime juice, but eating large amounts of fresh bear meat, remained in good health. The crew left on the ship, taking their daily lime juice and subsisting on canned and salted meat, came down with scurvy. This led to the theory that scurvy was caused by tainted meat. Thus, in 1913, the Antarctic explorer, Captain Scott, and his companions suffered miserable deaths because their expedition was provisioned on the basis of the tainted meat theory and carried no antiscorbutics.
Because of the lack of accurate knowledge concerning our elusive molecule, many other odd theories on scurvy were proposed, some as late as the 1910s when we should have known better. One theory claimed that scurvy was due to an "acid intoxication" of the blood, another that it was a bacterial autointoxication, and as late as 1916, someone "discovered" its bacterial origin. Probably the crowning nonsense was put forward in 1918, when it was claimed that constipation was the cause of scurvy. After World War I, two German doctors who had been assigned to care for Russian prisoners of war came up with the novel idea that scurvy was transmitted by vermin; apparently the Russians had both.
Aside from these blunders, the nineteenth century saw many great advances in the sciences. The germ theory of disease was established after much initial resistance from the medical dogma of the time. And progress was made in the scientific study of nutrition. A brief historical review of the science of nutrition will provide the reader with some background to better understand the theme of this book.
In the early years of the nineteenth century, experiments were conducted in which animals were fed purified diets of the then known food constituents: fats, carbohydrates, and proteins. But the animals did not thrive. Not only did they not grow well but they also developed an opacity of the cornea of the eye (which we now know is due to a vitamin A deficiency). Later in the century, in 1857 in Africa, Dr. Livingstone noted a similar eye condition in poorly fed natives. Later, about 1865, similar observations were made of slaves on South American sugar plantations. The condition was attributed to some toxic constituent in their monotonous diet rather than the lack of some element. Most of the investigators in the growing science of nutrition concentrated on learning the basic facts about calories and the utilization of fats, carbohydrates, and proteins in the diet. Why a purified diet, adequate in these elements, would not support life, long remained a mystery.
In the latter decades of the nineteenth century and early in the twentieth century, many important observations were made which helped unravel this mystery. The Japanese Tahaki showed that the disease beriberi, then afflicting 25 to 40 percent of the Imperial Japanese Fleet, could be prevented by adding meat, vegetables, and condensed milk to the customary diet of rice and fish. He missed the significance of his results because he believed the improvement was due to a higher calorie intake comparable to that of the German and British navies. In 1897, the Dutchman Eijkman, working in Batavia, was able to produce beriberi in chickens by feeding them polished rice (rice from which the husk coating is removed) and was able to cure the birds by giving them extracts of the husk or polishings. But he did not interpret his experiments correctly either. He thought that the polished rice contained a poison and the polishings contained a natural antidote. Four years later another Dutchman correctly interpreted these experiments, suggesting that beriberi in birds and men is caused by the lack of some vital substance in the polished rice that is present in the rice bran.
In 1905 and 1906, Pekelharing in Holland and Hopkins at Cambridge, England, repeated the old experiment of feeding rats and mice on purified diets and again found that they failed to grow and died young. But they went one step further and found that adding small amounts of milk, not exceeding four percent of the diet, allowed the animals to grow and live. Both investigators realized that there was something present in natural foods that is vitally important to good nutrition. The concept of deficiency diseases (the idea that a disease could be caused by something lacking in the diet) was dawning.
Two Norwegian workers, Holst and Frohlich, in 1907, were also investigating beriberi, which was common among the sailors of the Norwegian fishing fleet. They were able to produce the disease in chickens and pigeons but they wanted another experimental animal, a mammal, to work with. They chose the guinea pig, and a fortunate choice it was for man and the science of nutrition. After feeding the guinea pigs the beriberi-inducing diets, they found that the guinea pigs rapidly came down with scurvy instead. This was a startling discovery, because up to this point it was believed that man was the only animal that could contract scurvy. This was also a very valuable and practical contribution because now an experimental animal was available that could be used for all sorts of exact and quantitative studies of scurvy. It also showed that there was something very similar an unique about the physiology of the guinea pig and man. This simple observation was the greatest advance in the study of scurvy since the experiments of Lind in the 1740s. This discovery could have been made twelve years earlier had Theobald Smith, the famous American pathologist, realized the importance of his observation that guinea pigs fed a diet of oats developed a hemorrhagic disease. But he failed to relate the bleeding of his guinea pigs with human scurvy.
There were many brilliant workers in the field of nutrition, but we need only mention one other in the thread of occurrences that led to the present misconceptions regarding our elusive molecule. Casimir Funk, working in the Lister Institute, prepared a highly concentrated rice-bran extract for the treatment of beriberi and designated the curative substance in this extract as a "vitamine." In 1912-1913 he published his then radical theory that beriberi, scurvy, and pellagra and possibly rickets and sprue were all "deficiency diseases," caused by the lack of some important specific trace factor in the diet. Subsequent work divided these factors into three groups: vitamin A, the fat-soluble antiophthalmic factor; vitamin B, the water-soluble antineuritic substance; and vitamin C, the water-soluble antiscorbutic material. It was not known for many years whether each vitamin represented a single substance or many. Vitamin B was later found to consist of a group of chemically diverse compounds, while both vitamin A and vitamin C were single substances. Time added more "letters" to the vitamin alphabet. Eventually the different vitamins were isolated, purified, and their chemical structures determined and finally synthesized: but this took many years.
In the early decades of the twentieth century, ascorbic acid was still unknown. The sum total of our knowledge was about equal to Lind's but great changes were in store.
7
_____________________________________________________________
In 1907, with the discovery that guinea pigs were also susceptible to scurvy, experimental work heretofore impossible could be conducted in the study of the disease. Foods could be assayed to determine the amounts of antiscorbutic substance they contained. The general properties of our elusive molecule could be studied by using various chemical treatments on the antiscorbutic extracts and following them with animal assays to learn why the molecule was so elusive and sensitive.
The bulk of the experiments on scurvy in the early years of the twentieth century was carried out by nutritionists who had contrived the vitamin hypothesis and the concept of deficiency diseases. They had already taken scurvy under their wing as a typical dietary deficiency disease. They had even named its cause and cure, vitamin C, without knowing anything more definite than the fact that it was some vague factor in fresh vegetables and fruits.
While the nutritionists were busy with their rats, mice, guinea pigs, and vitamin theories, another important event took place in medicine. We will mention it briefly here because it happened at about this time, and we will come back to it later to explain its significance to ascorbic acid and scurvy.
In 1908, the great English physician, Sir Archibald Garrod, presented a remarkable series of papers in which he set forth new ideas on inherited metabolic diseases, or as he stated it, "Inborn errors of metabolism." These are diseases due to the inherited lack of certain enzymes. This lack may cause a variety of genetic diseases depending upon which particular enzyme is missing. The fatality of these diseases depends upon the importance of the biochemical process controlled by the missing enzyme. It may vary from relatively benign to rapidly fatal conditions. This was a revolutionary concept for those days -- a disease caused by a biochemical defect in one's inheritance.
Now getting back to the main thread of our story, the nutritionists continued their work with their new-found experimental animals and in the next decades uncovered more information about our elusive molecule.
To study the chemistry of a substance such as ascorbic acid, it is necessary to concentrate it from the natural extracts, to isolate it in pure form, to crystallize it, and to recrystallize it to make sure it is just one single chemical compound. Only then can it be identified chemically, and its molecular structure determined. Once the structure of the compound is known, it is relatively easy to find ways to synthesize it. Comparison of the synthetic material with those of the original crystals will either confirm or deny whether the chemist's tests and reasoning were correct.
In the early 1920s scientists began concentrating the vitamin C factor and studying its behavior under various chemical treatments by means of quantitative animal assays. It was a long drawn-out procedures but it was gradually becoming clear why the molecule vanished so easily. The real tests, however, had to wait its isolation and crystallization in pure form.
As scientists approached the home stretch of the search their pace quickened and attracted more workers. There was a group at the Lister Institute in England and another in the United States; a Russian directed group in France and later other groups formed. History has a way of playing tricks on the course of events. In the years before our elusive molecule was finally pinned down, there were a few close calls in the attempts to isolate it in pure form which, for one reason or another, were never successful. In the early 1920s a student worker at the University of Wisconsin, studying the biochemistry of oats, isolated a crude crystalline fraction which may have been our elusive material. The work was carried no further because the dean refused a research grant of a few hundred dollars which was required to pay for animal assays of these crude crystals. In 1925, two U.S. Army workers at Edgewood Arsenal were on the verge of obtaining crystals of the antiscorbutic substance when they were transferred to different stations; their work was never completed. Bezssonov, the Russian worker in France, may have isolated antiscorbutic crystals from cabbage juice in 1925, but for some unknown reason these crystals were never thoroughly investigated.
In 1928, Albert Szent-Gyorgyi, working at Cambridge, England, on a biochemical problem unrelated to scurvy or vitamin C, reported that he has isolated crystals of a new sugarlike substance with very unusual chemical properties from the adrenal gland of the ox. He called the substance "hexuronic acid." Similar crystals were isolated from oranges and cabbages. While these crystals were isolated in connection with another biochemical problem, Szent-Gyorgyi noted their similarity to the chemical reaction of vitamin C and suspected there was some connection. He made arrangements to have the crystals tested by animal assay but before he could obtain enough crystals he left England and the matter was left in abeyance. It was not until 1931, after he had resettled in Hungary, that he was able to pick up the threads and carry out the necessary tests which proved hexuronic acid to be vitamin C. In that year, a student of Hungarian descent, J.L. Svirbely, who had worked with the American team of vitamin C researchers at the University of Pittsburgh under Charles Glenn King, returned to Hungary and joined forces with Szent-Gyorgyi to work on the problem. The years 1932 and 1933 were very fruitful and saw many published reports by American, Hungarian, English, and other workers. All this research showed that hexuronic acid was indeed our elusive molecule, and it was soon renamed "ascorbic acid."
With the pure crystals of ascorbic acid available, its chemical structure was quickly determined and methods for its synthetic production from the sugars were devised. The development of these syntheses permitted unlimited production of ascorbic acid at a low price and provided a practical solution to a problem that had always beleaguered man -- but of which he was still unaware.
Thus our elusive molecule was finally pinned down and revealed. The search had ended. The importance of this work was recognized in 1937 by the award of two Nobel Prizes: one to Szent-Gyorgyi for his biochemical discoveries and the other to the English chemist, Sir Walter Haworth, for his research on the chemical structure and synthesis of ascorbic acid.
8
_____________________________________________________________
Although the search for our elusive molecule has ended, our story has not. Actually, the means for ascorbic acid's unlimited synthetic production provide us with a second beginning. We shall now see how the subsequent investigations of the following forty years got off to a wrong start which hindered our understanding of this unique molecule and the thorough exploitation of its vast therapeutic potentialities. To better understand the circumstances of this paradoxical situation, we will try to look into the mental climate of the most advanced investigators in this field in the early 1930s. A reader of the previous historical chapters would know vastly more about ascorbic acid and its place in human evolution than anyone in that era. The mechanisms of the natural synthesis of ascorbic acid by plants and animals were still unknown and their genetic significance was not even a gleam in any researcher's eye. The important enzyme L-gulonolactone oxidase was awaiting discovery in the far distant future and the importance of this and related enzyme systems were not even suspected. All the investigators had been brought up on the dogmatic tenets of the then thirty-year-old vitamin-C theory. Scurvy was an avitaminosis (a dietary deficiency disease) and this nutritional disturbance could be prevented or cured by ingesting minute amounts of this trace nutrient into the diet. Thus vitamin C was considered a trace food constituent found in certain foodstuffs and was not even remotely associated with the idea that it might have been a product of man's original metabolism.
In the early and mid-1930s the status of scurvy had changed little from the time of Lind in 1753 when it was considered a food-related disease, except that 20 years earlier someone had called the unknown substance a "vitamine." As time rolled one and more facts were gathered, it seemed that "vitamin C" was not behaving like a typical vitamin. For nearly all animals it was not even a "vitamin" because of its widespread manufacture in their own bodies; they never got scurvy no matter how little vitamin C was in their food. Out of the thousands of different animals existing in Nature only three (man, monkeys and guinea pigs) were known to need vitamin C in their foodstuff. The effective dosages of vitamin C were also considerably higher than those for the other known vitamins. As the chemical and enzymatic mechanisms of how plants and animals make their own vitamin C became known, the terms "vitamin C" and "ascorbic acid" became more and more synonymous. In the field of biochemical genetics, great advances were being made in understanding the mechanisms of heredity. A vast amount of clinical work was reported on the use of ascorbic acid in the treatment of all known diseases, much without spectacular success except in the case of scurvy. These developments and others, covering a quarter of a century, were instrumental in shaping the author's genetic concepts of the human need for ascorbic acid. The vitamin C hypothesis never attempted to explain why we were susceptible to scurvy, only how we got it. This hypothesis no longer served the new accumulation of facts, and clearly a new approach was required. However, up until 1966, the vitamin C hypothesis was an unquestioned part of published medical dogma.
For the development of these new concepts we have to return to Sir Archibald Garrod, mentioned in the last chapter, who in 1908 introduced the concept of the inherited enzyme disease into medicine. At the time, this was a revolutionary way of explaining the cause of disease in man. These genetic diseases are caused by the inherited lack or inactivity of a certain specific enzyme. The inability of the enzyme to function normally prevents the body from carrying out the biochemical process involved. This may cause toxic by-products to accumulate or abnormal biochemical pathways to develop which bring on the symptoms of the genetic disease. As mentioned earlier, the diseases range from the relatively harmless to those that are rapidly fatal. The reader will remember that the body contains thousands of enzymes for carrying out the living process and the absence of any one can bring on an "inborn error of metabolism." Since each of the body's enzymes is synthesized from a single gene in the chromosome, a slight mutation of the gene can cause the loss of an enzyme and thus cause a genetic disease.
Sir Archibald, in his original 1908 papers, described four genetic diseases but the list has now grown and new ones are constantly being reported. He reported on albinism, alkaptonuria, cystinuria and pentosuria, all due to the lack of a particular enzyme in the afflicted individual's biochemical inheritance. Albinism, a relatively harmless condition, is due to the lack of an enzyme used in the production of the black skin pigment, melanin. Alkaptonuria and cystinuria are both diseases of protein metabolism in which the missing enzyme causes an accumulation of intermediate protein digention products, which causes changes in the urine and other parts of the body. Alkaptonuria is relatively benign until late in life when it produces a severe type of arthritic condition. Cystinuria induces the formation of kidney and bladder stones, while the relatively rare and harmless pentosuria causes pentose, a sugar, to appear in the urine and may be confused with diabetes.
Like many other great discoveries in medicine, Garrod's work was almost ignored for a generation. In fact, an examination of the major genetics textbooks in use in 1940 fails to reveal any mention of alkaptonuria, described by Garrod thirty-two years earlier. Time has corrected this oversight and the importance of Garrod's pioneering work is now acknowledged by all.
Two more recent genetic diseases, now much in
the news, will be briefly mentioned: galactosemia, which afflicts
infants, is caused by a missing enzyme,
galactose-1-phosphate-uridyltransferase, (all enzyme names end with
"ase") that prevents babies from properly digesting the sugar in
milk. Unless they are promptly taken off a milk diet, they will sicken
and may die. Those that survive will be stunted in growth, may develop
cataracts, and may be mentally retarded. The other genetic disease,
phenylketonuria (or PKU), is another disease of infants and is caused by the
inherited lack of the enzyme, phenylalanine hydroxylase, producing a profound
disturbance of protein digestion. Unless the victims are placed on
special low-protein diets, irreversible brain damage can occur as well as
mental retardation and other nervous disorders.
Now back to ascorbic acid. In mammals, ascorbic acid is produced in the liver from blood glucose by the stepwise reactions shown in Figure 3. Each step, except the last, is controlled by a specific enzyme. In the last step, the 2-keto-L-gulonolactone, once formed, is automatically converted into ascorbic acid. No enzyme is required. On the right side of the diagram, the step of transforming L-gulonolactone into 2-keto-L-gulonolactone is catalyzed by the enzyme L-gulonolactone oxidase. This is the critical enzyme for humans who, because they carry a defective gene, cannot produce an active enzyme. This is the gene that mutated in a primate ancestor of ours millions of years ago. It is this step that is blocked in man and prevents him from producing large amounts of ascorbic acid from glucose in the liver.
Here we have the classic conditions for a genetic, missing-enzyme disease, and yet for years these simple facts have been ignored and scurvy has continued to be regarded as avitaminosis. In 1966, this author published the paper "On the Genetic Etiology of Scurvy," in which the history and pertinent facts were reviewed and it was pointed out that in scurvy we were dealing with a genetic, liver-enzyme disease and not simply a dietary disturbance. Since it is the prerogative of the discoverer to name a new disease, the author called it "hypoascorbemia" because low levels of ascorbic acid in the blood are characteristic of this disease.
This genetic approach now provides a natural rationale for the use of large amounts of ascorbic acid which served so well in the survival of the mammals in the course of evolution. Its implications for health and well-being are vast because it furnishes the basis for the new unexplored fields of preventive medicine and therapeutics (megascorbic prophylaxis and megascorbic therapy). It is hoped that the publication of these new ways of using ascorbic acid will stimulate the flood of research similar to that which occurred when ascorbic acid was discovered in the early 1930s. Let us see how long it will take to break down the "vitamin-barriers" of current orthodox medical dogma.
9
_____________________________________________________________
At this point it is best to discuss briefly some of the effects of ascorbic acid on various important bodily functions. This will give the reader a better insight and background for the later chapters. Ascorbic acid is involved in so many vital biochemical processes and is so important in daily living that, after forty years of research, we still have no clear idea of all the ways in which it works.
Throughout the evolution of the vertebrates, including the mammals, Nature has used ascorbic acid to maintain physiological homeostasis. In simple nontechnical terms, this means that when stressful situations arose which disturbed the biochemical equilibrium of the animal, ascorbic acid was produced in increased quantities to get things running normal again. The amount of ascorbic acid produced is related to the severity of the stresses and if enough was produced soon enough, then the animal was able to survive the bad biochemical effects of the stresses. If, however, the enzyme system for producing ascorbic acid was overwhelmed or poisoned by the stresses and too little ascorbic acid was produced, then the animal succumbed. Man, unable to produce his own ascorbic acid, could not take advantage of this natural protective process. Instead stresses only further depleted his low stores of this vital metabolite. Now he can easily duplicate this time-tested defensive mechanism by reaching for the bottle of ascorbic acid and swallowing additional quantities whenever he is subjected to biochemical stresses. In duplicating this normal process for combating stresses, man has one great advantage over the other mammals -- he can get an unlimited supply of ascorbic acid without being dependent upon an enzyme system which may not produce enough, quickly enough. All man needs to know is how much to take.
One of the outstanding attributes of ascorbic acid is its lack of toxicity even when given in large doses over long periods of time. This has been recognized since the 1930s, and ascorbic acid can be rated as one of the least toxic substances known of comparable physiological activity. It can be administered in huge doses, intravenously, without registering any serious side effects. Because of human variability and because the human organism has been exposed to such low levels of this essential substance for so long, some usually transient side effects may occur in a small percentage of hypersensitive individuals. This may be evidenced as diarrhea or rashes which clear up on lowering the dosage. In many cases it is possible to avoid these reactions by building up to the desired dosage gradually, which permits the body to become accustomed to these essentially normal mammalian levels. Taking the ascorbic acid with food or before meals often helps.
Chemically, ascorbic acid is a rather simple carbohydrate related to the blood sugar, glucose (see Figure 1, page __). Unlike glucose, it contains an unusual, highly reactive combination of molecules called the "ene-diol group." The presence of this group confers upon the ascorbic acid molecule certain unique biochemical characteristics which amy explain its vital importance in the living process. It transforms a relatively inactive sugar into a highly reactive, labile, and reversible carbohydrate derivative which readily donates or accepts electrons from its surrounding medium. This is know technically as an "oxidation-reduction system."
On a molecular basis, the whole living process is nothing more than an orderly flow and transfer of electrons. Therefore, having an abundance of a substance like ascorbic acid present in living matter makes this orderly flow and transfer of electrons proceed with greater ease and facility. It acts substantially like an oil for the machinery of life. This was discovered by Nature billions of years ago. Recent work indicates that this oxidation-reduction system can form even more active free radicals which may explain some of its unusual biological effects.
There are no large storage depots for ascorbic acid in the body and any excess is rapidly excreted. When saturated, the whole body may only contain 5 grams. This means that the body requires a continuous supply to replenish losses and depletions. The livers of nearly all mammals are constantly making and pouring ascorbic acid into their bloodsteams, but man's liver is unable to do this. He needs a constant, large, outside supply to make up for this genetic defect. When the different organs and tissues are analyzed, it is found that ascorbic acid concentrates in the organs and tissues with high metabolic activity; the adrenal cortex, the pituitary gland, the brain, the ovaries, the eyes, and other vital tissues. Any form of biochemical stress or physical trauma will cause a precipitous drop in the ascorbic acid levels of the body in general, or locally in the affected organs or tissues. In animals biochemically equipped to produce their own ascorbic acid, any stressful situation causes them to synthesize more and greater amounts to replace that destroyed or utilized in combating the stresses.
One of the most important biochemical functions of ascorbic acid in the body's chemistry is the synthesis, formation, and maintenance of a proteinlike substance called collagen. Collagen cannot be formed without ascorbic acid, which is absolutely essential to collagen production by the body. Collagen is the body's most important structural substance. It is the ground substance, or cement, that supports and holds the tissues and organs together. It is the substance in the bones that provides the toughness and flexibility and prevents brittleness. Without it the body would just disintegrate or dissolve away. It comprises about one-third of the body's total weight of protein and is the most extensive tissue system. it is the substance that strengthens the arteries and veins, supports the muscles, toughens the ligaments and bones, supplies the scar tissue for healing wounds and keeps the youthful skin tissues soft, firm, supple and wrinkle free. When ascorbic acid is lacking, it is the disturbance in collagen formation that causes the fearful effects of scurvy -- the brittle bones that fracture on the slightest impact, the weakened arteries that rupture and hemorrhage, the incapacitating muscle weakness, the affected joints that are too painful to move, the teeth that fall out, and the wounds and sores that never heal. Suboptimal amounts of ascorbic acid over prolonged periods during the early and middle years, by its effect of producing poor quality collagen, may be the factor in later life that causes the high incidence of arthritis and joint diseases, broken hips, the heart and vascular diseases that cause sudden death, and the strokes that bring on senility. Collagen is intimately connected with the entire aging process.
Ascorbic acid has a marked activating effect on many bodily enzymes and makes the processes controlled by these enzymes proceed at a more favorable rate. It is very important in nutrition, the digestion of food and the biochemistry of the body's utilization of carbohydrates, proteins, and fats. In carbohydrate metabolism it has a pronounced activating effect on insulin. It is essential to the proper functioning of the nervous system. Brain chemistry is dependent on the maintenance of proper levels of ascorbic acid and high levels are essential in treating nervous and mental disorders, as we shall see in a later chapter.
Ascorbic acid is a potent detoxicant which counteracts and neutralizes the harmful effects of many poisons in the body. It will combat various inorganic poisons, such as mercury and arsenic, and it neutralizes the bad reactions of many organic poisons, drugs, and bacterial and animal toxins. Ascorbic acid detoxifies carbon monoxide, sulfur dioxide, and carcinogens, so it is the only immediate protection we have against the bad effects of air pollution and smoking. It has also been shown that ascorbic acid increases the therapeutic effect of different drugs and medicines by making them more effective. Thus, less of a drug is required if it is taken in combination with large amounts of ascorbic acid. Diabetics could reduce their insulin requirements if this were practiced. Even an aspirin should be accompanied by a large does of ascorbic acid to heighten its analgesic effect and lessen its toxic action on the body.
Ascorbic acid in large doses is a good nontoxic diuretic. A diuretic is a substance that stimulates the excretion of urine. Thus, ascorbic acid at proper dosage levels will drain waterlogged tissues and reduce accumulated water in the body in heart and kidney diseases.
The antiseptic and bactericidal qualities of ascorbic acid have long been known. At relatively low levels it will inhibit the growth of bacteria and at slightly higher amounts it will kill them. The bacteria causing tuberculosis is particularly sensitive to the lethal action of ascorbic acid.
One of the body's defenses against bacterial infections is the mobilization of white blood cells into the affected tissues. The white blood cells then devour and digest the invading bacteria. This process is known as phagocytosis and is controlled by ascorbic acid. The number of bacteria that each white blood cell digests is directly related to the ascorbic acid content of the blood. This is one of the reasons why a lack of ascorbic acid in the body produces lowered resistance to infectious diseases.
Ascorbic acid is also a potent and nonspecific virucide. It has the power to inactivate and destroy the infectivity of a wide variety of disease-producing viruses including poliomyelitis, herpes, vaccinia, foot-and-mouth disease, and rabies. It only does this, however, at relatively high doses, not a "vitamin" level.
There is a relationship between ascorbic acid and the production and maintenance in the body of the adrenal cortical hormones. The adrenal gland, where this hormore is produced, also happens to be the tissue where the highest concentration of ascorbic acid is found.
In 1969 it was reported that laboratory tests conducted at the National Cancer Institute showed that ascorbic acid was lethal to certain cancer cells and harmless to normal tissue. This might be the long awaited breakthrough in cancer therapy. Intensive study and research should immediately be concentrated to investigate these possibilities.
This has been a brief and incomplete summary of ascorbic acid's many biochemical functions and of its vital importance in keeping the body in good operating condition. Even this incomplete review should not only give the reader an idea of the many important functions of ascorbic acid, but also leave the very distinct impression that ascorbic acid can be of much greater use to man than as a mere prevention of clinical symptoms of scurvy.
10
_____________________________________________________________
No one would have any difficulty recognizing the violent, extreme symptoms of totally "uncorrected" hypoascorbemia -- the clinical scurvy; but the milder forms, from which most people suffer, are difficult to detect. Chronic hypoascorbemia, or as it was previously called, "subclinical scurvy," is relatively symptom-free and can only be diagnosed by clinical or chemical testing, or by difficult long-term observations. Acute scurvy in the well-developed nations is, nowadays, not a common disease for two reasons. First, the daily amounts of ascorbic acid needed to protect against the symptoms of clinical scurvy are very small and, second, the improvements in food preservation and distribution make it easy to obtain these small amounts in foods available the year round. This i snot the case, however, for chronic hypoascorbemia. Anyone who depends solely on foodstuffs for ascorbic acid cannot expect "full correction" of hypoascorbemia. The more stress that such an individual is under, the higher would be the deficit. It is the lack of recognition of the distinction between acute scurvy and chronic hypoascorbemia, and the narrow aims of the "vitamin" theory, that have given a false sense of security, for the past sixty years, as to the adequacy of foodstuffs to fully supply the body's needs for ascorbic acid.
Hypoascorbemia can be "corrected" by supplying the individual with ascorbic acid in the amounts the liver would be making and supplying to the body if the enzyme were not missing. How, then, can we determine the amounts of ascorbic acid the human liver would be producing by an enzyme that is not there? The solution to this question may not be as difficult as it may seem at first glance. If the requirements for ascorbic acid in man are assumed to be similar to those of other, closely related mammals, then, by measuring the amounts of ascorbic acid produced by other mammals, we should be able to get a pretty good estimate of what man would be making, had he the complete synthetic enzyme system.
When we look for this very important data in the literature, it is amazing how little we find. the only information available is on the rat. No one has bothered to determine the amounts of ascorbic acid the larger mammals such as the pig, dog, or horse are capable of producing.
Clearly, a great deal more research is required to determine the extent of ascorbic acid synthesis by different mammals so that a more accurate estimate of man's needs can be calculated. Until this work is completed, we are forced to rely on the figures presently available for the rat.
Form these available figures, "full correction" of hypoascorbemia in a 70-kilogram adult human is estimated to require a daily intake of 2,000 to 4,000 milligrams (2.0 to 4.0 grams) of ascorbic acid, under conditions of little or no stress. Under conditions of stress, the data indicates an increase to about 15,000 milligrams (15.0 grams) per day. Under very severe stresses, even more may be required.
Biochemical stress covers a wide range of conditions, among which may be mentioned: bacterial and viral infections, physical trauma, injuries and burns, exposure to heat, cold, or noxious fumes, ingestion of drugs and poisons, air pollution and smoking, surgery, worry, aging, and many others.
Ascorbic acid is rapidly absorbed from the digestive tract so that "full correction" can be established by giving it, preferably in solution, in several oral does during the day. This is easily and pleasantly accomplished by dissolving a one-half level teaspoonful of ascorbic acid powder (1,500 milligrams or 1.5 grams) in a half glass of fruit or tomato juice or in about two ounces of water sweetened to taste. A dose in the morning and another at night and possibly one midday should establish "full correction" under conditions of no unusual stresses. This basic regimen for "normal" individuals should be the subject matter of extensive long-term clinical trials by statistically sufficient numbers of subjects of different age groups, to determine the long-range effects of these "corrective" dosage levels of ascorbic acid on their well-being, disease resistance, disease morbidity, the inhibiting effects on aging, and the possible lengthening of the human life span. The medical authorities supervising this proposed research, if and when it is conducted, should be convinced of its safety because: 1. These are normal mammalian ascorbic acid levels, 2. ascorbic acid virtually lacks toxicity, and 3. countless generations of monkeys have been raised using these ascorbic acid levels throughout their lives with the diet recommended by the National Research Council of the National Academy of Sciences.
Under conditions of biochemical stress, the frequency an size of the doses are increased depending upon severity of the stress. Amounts over 100 grams (100,000 milligrams) per day have been suggested for the therapy of acute viral infections. Clinical trials and dosages for the therapy of specific conditions will be discussed in later chapters.
The known lack of toxicity of ascorbic acid would indicate that no general serious side effects or toxic reactions would be incident to these "full correction" regimens. One mild discomfort that has been noted is diarrhea in individuals whose digestive tract is hypersensitive to the cathartic effect of fruit acids. The diarrhea stopped when the dosage was reduced and no other sequela resulted. Administration by injection has been used, but the oral route is so much simpler and more pleasant that intravenous doses may be reserved for cases where the oral route is not feasible or very severe stresses require heroic measures for building high blood levels of ascorbic acid quickly under medical supervision.
If gastric distress is encountered due to the acidity of the ascorbic acid, partial neutralization with small amounts of sodium bicarbonate or the use of sodium ascorbate instead of ascorbic acid will overcome this (see Chapter 21).
"Full correction" of this genetic disease has only been possible since the late 1930s when the synthetic production of ascorbic acid made it available in unlimited quantities at a low enough price. This "correction" could never be established by dependence upon ascorbic acid-containing foodstuffs because it is just physically impossible to ingest the large volumes of foods required to give the necessary dosage levels.
Actually this "full correction" concept merely attempts to duplicate in man a normal physiological process which is taking place all the time in other mammals, and that is to supply ascorbic acid in amounts in accordance with the needs.
The National Research Council of the National Academy of Sciences publishes reports from their Food and Nutrition Board and their Committee on Animal Nutrition. These are published as bulletins, available to the public, and they are the authoritative, last word on the nutrient requirements of humans and animals. The Food and Nutrition Board's bulletin on human needs is entitled "recommended Dietary allowances" (Seventh Revised Edition, 1968) and gives the recommended daily allowance for an adult human for ascorbic acid as 60 milligrams per day (about one milligram per kilogram of body weight). From the Committee on Animal Nutrition's "Nutrient Requirements of Laboratory Animals" (1962) we find some startling figures. The recommended diet for the monkey -- our closest mammalian relative -- is 55 milligrams of ascorbic acid per kilogram of body weight or 3,830 milligrams of ascorbic acid per day for the average adult human. The daily amount suggested as adequate for the guinea pig varies depending upon which of two diets is selected and ranges from 42 to 167 milligrams per kilogram of body weight (based on a 300-gram guinea pig). This amounts to 2,920 milligrams to 11,650 milligrams per day for the average adult human.
In summary, on an equivalent body weight basis the daily intake of ascorbic acid recommended by the National Research Council for humans is 60 milligrams; for monkeys, 3,830 milligrams; and for guinea pigs, 3,920 to 11.650 milligrams. It is noteworthy that the figure for the monkeys is similar to our estimate of the daily amount that would be produced in the human liver if the final essential enzyme were not missing. There is a 55-fold difference between the amount recommended for man and that given for monkeys; and the guinea pigs have a 42- to 167-fold advantage over man. Are these agencies shortchanging the human population in favor of laboratory animals? The pressure groups that are continually complaining of how badly laboratory animals are being treated certainly would have no complaint on this score. It is time we had a pressure group to see that humans also receive enough ascorbic acid!
The author has not been alone in the belief that the present recommended levels of ascorbic acid may not be the optimal levels to fulfill all our requirements. In 1949, Geoffrey H. Bourne, now head of the Yerkes Regional Primate Research Center in Atlanta, Georgia, pointed out that an adult gorilla in the wild state consumes about 4.5 grams of ascorbic acid a day in his food. He also speculated that the recommended milligrams a day for humans might be wide of the mark and 1 or 2 grams a day might be the correct amount.
Dr. Albert Szent-Gyorgyi, who received the Nobel Prize in Medicine for his research on ascorbic acid, in a private communication to the author in 1965, stated that what he liked about the author's genetic concept is that it suggested " that the daily dosage of ascorbic acid in man should be much higher. I have always pleaded for such a higher dosage."
Dr. Frederick R. Klenner of Reidsville, North Carolina, who has had more actual clinical experience in megascorbic prophylaxis and megascorbic therapy in the past thirty years than anyone else in the world, routinely prescribes ten grams of ascorbic acid daily to his adult patients for the maintenance of good health. His daily dosage schedule for children is one gram of ascorbic acid per year of age up to ten years and ten grams daily thereafter (e.g. a four-year-old child would receive four grams daily).
Linus Pauling pioneered in the field of molecular diseases with the discovery, published in 1949, that sickle-cell anemia is due to slight, but very important, changes in the structure of the blood protein, hemoglobin. He has also been very active in developing concepts which indicate that we may have inadequate levels of various natural substances normally present in the body, and which can bring forth symptoms of disease. In 1967, in a communication to the Thirteenth International Convention on Vital Substances, Nutrition, and Diseases of Civilization, held in Luxumbourg, Dr. Pauling described other molecular diseases and developed the concept of "orthomolecular therapy." Generally, orthomolecular therapy involves supplying vitamins, amino acids, or other natural bodily constituents which are at suboptimal levels by intakes of large amounts of the needed substance.
Dr. Pauling also described in his paper the application of orthomolecular medicine to the treatment of mental disease by the provision of high levels of ascorbic acid and other vitamins as the preferred method of treatment. The subject of orthomolecular psychiatry was developed in further detail in a 1968 paper appearing in the April 19, 1968 issue of Science. In the book Vitamin C and the Common Cold published in 1970, Dr. Pauling devotes a chapter to orthomolecular medicine. The use of high levels of ascorbic acid in the prevention and treatment of the common cold is a practical application of the principles of orthomolecular medicine. Megascorbic prophylaxis and megascorbic therapy are, thus, branches of orthomolecular medicine.
In a paper presented by Dr. Pauling to the National Academy of Sciences and appearing in the December 15, 1970 issues of their Proceedings, calculations were made from the caloric and ascorbic acid content of raw plant foods. From this data, Dr. Pauling concluded that the optimum daily intake of ascorbic acid, for most human adults, is in the range of 2.3 grams to 9 grams. Because of the variation due to "biochemical individuality" the range of optimum intake for a large population may be as high as 250 milligrams to 10,000 milligrams (10 grams) or more a day.
"Biochemical individuality" is a concept from the work of Professor Roger J. Williams at the University of Texas, which indicated that individuals vary over a considerable range in the need and use of metabolites and that a value based on a so-called average may be far off the mark.
Dr. Leon E. Rosenberg, Associate Professor of Pediatrics and Medicine at the Yale University School of Medicine, in discussing biochemical abnormalities due to hereditary defects, suggested differentiating between vitamin-deficiency diseases and vitamin-dependent diseases. The vitamin-dependent diseases are those which may require 10 to 1,000 times the "normal" daily requirements for their successful treatment. Rosenberg's work was confined to vitamin-dependent genetic defects of the various B vitamins and vitamin D. He apparently has not worked with ascorbic acid.* *(This was reviewed in Science News of August 29, 1970, pages 157-158, and in the Journal of the American Medical Association of September 21, 1970, page 2001.)
The interesting conclusion which can be drawn from: 1. the gorilla data of Bournes, 2. the evolutionary raw-plant food calculations of Pauling, 3. the daily synthesis of ascorbic acid by the rat, 4, the dietary recommendations of the National Research Council for the good nutrition of monkeys, and 5. the actual human clinical data of Klenner, is that all of these point to an intake of several grams a day, rather than the sixty milligrams a day now regarded as adequate.
PART II
____________________________________________________________
Pathways to Research
11
____________________________________________________________
BREAKING THE 'VITAMIN' BARRIER
The discovery of ascorbic acid and its identification as the anti-scorbutic substance vitamin C in 1933 literally launched thousands of medical research projects on practically every known disease and pathologic condition. Here was a newly discovered substance of extremely unusual medicinal properties with almost magical curative properties for scurvy. A person at death's door from scurvy could be miraculously returned to health in a few days with a few specks of ascorbic acid.
The number of medical research papers published and the variety of diseases covered in the flood of research triggered by this discovery were to great that five years later, in 1938, a contemporary author remarked, "So many papers have now been published on this subject that it is difficult to find a single ailment to which the human or animal body is prone that has not been investigated." In 1938 and again in 1939, over six hundred medical research papers on ascorbic acid were published throughout the world.
In reviewing this tremendous volume of medical literature, one is struck by the dominating influence that the nutritional and vitamin aspects of ascorbic acid had on most of these medical investigators. This was due to their thorough indoctrination in the vitamins C hypothysis. For them, the antiscorbutic substance could only be a vitamin, and scurvy was just a dietary irregularity. They also knew that the merest trace of ascorbic acid, a few milligrams a day, would serve as a curative dose for scurvy. Thus, when they tackled other diseases in the early 1930s, they used dosage levels found satisfactory for scurvy. While many of the therapeutic results indicated encouraging good effects, just as many showed a lack o this low-dosage bias that throws grave doubts on the usefulness of the clinical results reported over the next several decades.
These early investigators also had practical reasons for using the low-dosage levels. In the early 1930s, ascorbic acid was a relatively rare and expensive commodity which was rationed by the early sources of supply. Investigators were limited by availability and could not have given bigger doses,even if they had wanted to . In the late 1930s, scarcity was not longer a problem since large-scale synthetic production was getting underway and there was a substantial drop in prices. But the low-dosage tests continued.
In those early days, therapeutic doses of 50 to 100 milligrams per day were considered "large" and by vitamin criteria they were. It is thoroughly disheartening, however, to go through the later literature and find in paper after paper, in spite of the early lack of success,the continued use of these low-dosage levels. These workers repeated and repeated the early mistake of using ineffective, small dosages. Hardly anyone was inspired to increase the dosages and test higher levels to see if they were more effective. This situation is even stranger when we remember that ascorbic acid is a substance with virtually no toxicity, so there was no danger from a substantial dosage increase. These workers were so imbued with the "vitamin" mental block prevented them from applying the common principle of pharmacology of adjusting the dosage level t get the effect desired. They thought of ascorbic acid as a "vitamin" and as a "vitamin" they expected miracles from trace amounts. What was needed was "medication," not "alimentation," but this simple fact escaped the majority of investigators.
Successful therapy with ascorbic acid was reported, but only in the work of a few investigators who used sufficiently high dosages of many grams per day. These rare individuals were the ones who provided the foundations of megascorbic therapy, which have to be more thoroughly extended and explored. The genetic disease concept now provides a clear and definite rationale for the use of these multigram doses.
One of the few clinical investigators who realized the importance of dosage levels in ascorbic acid therapy was Dr. F.R. Klenner,who, in the late 1940s and early 1950s, successfully pioneered the remarkable and dramatic therapeutic results in diseases such as poliomyelitis, still largely ignored by medicine, will be discussed in the chapter on viral diseases, but let us read his views on the work preceding his studies.
A review of the literature in preparation of this paper, however, presented an almost unbelievable record of such studies. The years of labor in animal experimentations, the cost in human effort and in "grants" and the volumes written, make it difficult to understand how so many investigators could have failed in comprehending the one thing that would have given positive results a decade ago. This one thing was the size of the dose of vitamin C employed and the frequency of its administration. No one would expect to relieve kidney colic with a five-grain aspirin tablet; by the same logic we cannot hope to destroy the virus organism with doses of vitamin C of 10 to 400 milligrams. The results which we have reported in virus diseases using vitamin C as the antibiotic may seem fantastic. These results, however, are no different from the results we see when administering the sulfa, or mold-derived drugs against many other kinds of infections. In the latter instances we expect and usually get forty-eight- to seventy-two hour cures; it is laying no claim to miracle working, then, when we say that many virus infections can be cleared within a similar time limit [with ascorbic acid] (*These comments are contained in his 1949 paper entitled, "The Treatment of Poliomyelitis and Other Virus Diseases with Vitamin C.")
This lone voice has been echoing, unheeded,through the maze of medical literature for nearly two decades, while the still-unsuccessful search for another anti-viral agent still goes one.
The following chapters will briefly summarize the clinical experiences, as reported in the medical literature of the past forty years on ascorbic acid therapy of a wide variety of diseases. Provocative ideas and results will be pointed out and further lines of research with the new genetic concepts in mind, and to start long-term investigations of these inadequately explored areas. It will be necessary to break down the sixty-year-old vitamin C mental barriers that have impeded research thus far and to apply some logic to the protocols of clinical research. Ascorbic acid has been thoroughly tried at nutritional levels, over the years, without notable success. It is high time that megascorbic levels become the subject of clinical testing.
Before embarking on the large-scale clinical trials urged in later chapters, further information and additional tests should be conducted to resolve questions in three areas. First of all, the data on the estimated daily production of ascorbic acid in man is based on the results of tests on rats. It would be desirable to obtain additional data on the daily synthesis of ascorbic acid in the larger mammals under varying conditions of stress. In this way we would get a closer estimate of what man would be producing in his liver, if he did not carry the defective gene for L-gulonolactone oxidase.
Secondly, even though ascorbic acid is rated as one of the least toxic materials, man has been exposed to such low levels of it for so long that suddenly taking comparatively large amounts, orally, may provoke side reactions in a small percentage of certain hypersensitive individuals. Ascorbic acid in the mammals is normally produced in the liver and then poured directly into the boodstream. This completely avoids the digestive tract, which is normally the route for man. Evidence for these side reactions may be the appearance of gastric distress, vomiting, diarrhea, headache, or skin rashes, all of which disappear on reducing or eliminating the ascorbic acid. Tests should be conducted on these hypersensitive individuals to determine whether their symptoms can be avoided or controlled by substituting the non-acidic sodium ascorbate, by taking the doses with meals, or by gradually building up to the required dosage instead of initially prescribing and starting with the full dosage. In many cases, an initial intolerance to ascorbic acid disappears.
Thirdly, research should be instituted to determine the validity of the criticism of stone formation as a consequence of high ascorbic acid intakes. This criticism has been leveled in spite of the fact that these high levels have been normal for the mammals for millions of years and the fact that stone formation has been attributed to a lack of ascorbic acid (see also Chapter 22). The effect of increased diuresis, due to megascorbic levels, should also be investigated in relation to the mineral metabolism of sodium, potassium, calcium, and magnesium to determine whether increased levels of intake of these essential minerals are required and whether this regimen improves the body's tolerance to sodium.
It is hoped that the research pathways and protocols outlined the following chapters will serve as a guide for future clinical research to exploit the full therapeutic potential of ascorbic acid for the benefit of man. It is certainly not the intent or the desire of the author that the details of these research proposals be used as the basis for furthering self-medication in any form.